2-octenol | 22104-78-5
中文名称
——
中文别名
——
英文名称
2-octenol
英文别名
2-octen-1-ol;oct-2-en-1-ol
CAS
22104-78-5
化学式
C8H16O
mdl
——
分子量
128.214
InChiKey
AYQPVPFZWIQERS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-17.5°C (estimate)
-
沸点:202.67°C (estimate)
-
密度:0.8490
-
LogP:2.674 (est)
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:9
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:使用支持的单体钒-氧化物催化剂简单高效地进行烯丙醇的1,3-异构化摘要:由高五小组晋升:二氧化硅负载的单体钒氧化物促进了各种烯丙基醇的异构化,包括在放大和无溶剂的反应条件下。该催化剂还显示出高的可重复使用性,而活性没有下降。DOI:10.1002/cctc.201300200
-
作为产物:参考文献:名称:带有 AcAc 型配体的二氧钼配合物的合成、表征和催化行为摘要:一系列[MoO2(acac')2][acac'=乙酰丙酮基型配体:二苯甲酰甲烷(3)、1-苯甲酰丙酮(4)、双(对甲氧基苯甲酰)甲烷(5)、2-乙酰环戊酮(6), 2-乙酰环己酮 (7) 和 2-乙酰基-1-四氢萘酮 (8)] 配合物已通过使用钼酸钠和所需的 acac 型配体作为起始材料的简单合成方法以 44-83% 的产率合成。所有配合物均通过 IR、UV/Vis、NMR 和高分辨率 ESI-MS 进行表征,对于化合物 3、4 和 8,通过 X 射线衍射获得固态结构。所有配合物都含有顺式二氧钼部分,这可以通过 IR 光谱中特征性的 Mo=O 振动和带有不对称配体(4 和 6-8)的配合物的 NMR 光谱中出现的四组信号来证明,并且由固态结构证实。发现这些配合物在使用工业级甲苯作为溶剂在 100 °C 的空气中将 1-苯基乙醇脱水成苯乙烯的过程中作为催化剂具有活性。[MoO2{(tBuCO)2CH}2]DOI:10.1002/ejic.201201350
文献信息
-
Chemoenzymatic one-pot reaction of noncompatible catalysts: combining enzymatic ester hydrolysis with Cu(<scp>i</scp>)/bipyridine catalyzed oxidation in aqueous medium作者:Henning Sand、Ralf WeberskirchDOI:10.1039/c7ra05451c日期:——chemoenzymatic one-pot reactions in aqueous media remain challenging and are limited today to metal-catalysts that display high activity in aqueous media. Here, we report the first combination of two incompatible catalytic systems, a lipase based ester hydrolysis with a water-sensitive Cu/bipyridine catalyzed oxidation reaction, in a one-pot reaction in aqueous medium (PBS buffer). Key to the solution在过去的几年中,一锅反应中化学催化剂和生物催化剂的结合引起了人们的极大兴趣。然而,由于每种催化剂需要非常不同的反应条件,因此在水性介质中的化学酶一锅法反应仍然具有挑战性,并且今天仅限于在水性介质中表现出高活性的金属催化剂。在这里,我们报告了在水介质(PBS缓冲液)的一锅反应中,两种不相容的催化系统的第一个组合,即基于脂肪酶的酯水解与水敏性Cu /联吡啶催化的氧化反应。解决方案的关键是将铜/联吡啶催化剂分隔在核壳状纳米颗粒中。我们显示了铜/联吡啶官能化的纳米粒子的合成和表征,以及在水性介质中烯丙基和苄基醇的氧化中的应用。此外,该工作证明了第一步反应过程的实施,该过程具有优化的反应条件,其中包括在第一步中使用脂肪酶(CAL-B)水解各种乙酸酯底物,然后在好氧条件下将所得醇氧化为相应的醛在水性介质中的条件。
-
The formyloxyl radical: electrophilicity, C–H bond activation and anti-Markovnikov selectivity in the oxidation of aliphatic alkenes作者:Miriam Somekh、Mark A. Iron、Alexander M. Khenkin、Ronny NeumannDOI:10.1039/d0sc04936k日期:——outer-sphere electron transfer between the formyloxyl radical donor and the [CoIIIW12O40]5− polyanion acceptor forming a donor–acceptor [D+–A−] complex is proposed to induce the observed anti-Markovnikov selectivity. Finally, the overall reactivity of HC(O)O˙ towards hydrogen abstraction was evaluated using additional substrates. Alkanes were only slightly reactive, while the reactions of alkylarenes过去,很少通过实验观察到甲酰氧基自由基HC(O)O 3,这些研究是在电子结构的背景下进行的理论光谱分析。缺乏方便的制备甲酰氧基自由基的方法,使得人们无法研究其对有机底物的反应性。最近,我们发现在甲酸/甲酸锂的阳极电化学氧化中形成了HC(O)O 3。使用[Co III W 12 O 40 ] 5-聚阴离子催化剂,可导致由苯形成甲酸苯酯。在这里,我们介绍我们对电化学原位反应性的研究用有机底物生成HC(O)O 3。根据实验和计算得出的哈米特线性自由能关系,与苯的反应和选择的取代衍生物表明,HC(O)O 3是亲电子的。HC(O)O 3与末端烯烃的反应明显有利于抗马尔可夫尼科夫氧化反应,产生相应的醛作为主要产物以及进一步的氧化产物。以1-己烯为代表的底物进行的可能的反应路径分析,有利于从烯丙基C–H键中抽出氢形成己烯丙基的可能性,然后强烈建议在C1位置进一步进攻第二个HC(O)O˙自由基。据推测,进一步的氧化产物主要是由于HC(O)O
-
Direct Catalytic Enantioselective Benzylation from Aryl Acetic Acids作者:Patrick J. Moon、Zhongyu Wei、Rylan J. LundgrenDOI:10.1021/jacs.8b11390日期:2018.12.19We demonstrate that metal-catalyzed enantioselective benzylation reactions of allylic electrophiles can occur directly from aryl acetic acids. The reaction proceeds via a pathway in which decarboxylation is the terminal event, occurring after stereoselective carbon-carbon bond formation. This mechanistic feature enables enantioselective benzylation without the generation of a highly basic nucleophile
-
Synthesis of Diastereomerically and Enantiomerically Pure 2,3-Disubstituted Tetrahydrofurans Using a Sulfoxonium Ylide作者:Jennifer M. Schomaker、Veera Reddy Pulgam、Babak BorhanDOI:10.1021/ja0469075日期:2004.10.1via Sharpless asymmetric epoxidation chemistry, offer access to a wide variety of enantiomerically pure compounds. In this communication, we describe the use of a Payne rearrangement to control regioselectivity in the ring-opening of a series of 2,3-epoxy alcohols with dimethylsulfoxonium methylide to yield diastereomerically and/or enantiomerically pure disubstituted tetrahydrofuran rings. The factors
-
Chemoselective hydrogen peroxide oxidation of allylic and benzylic alcohols under mild reaction conditions catalyzed by simple iron-picolinate complexes作者:Shinji Tanaka、Yoshihiro Kon、Takuya Nakashima、Kazuhiko SatoDOI:10.1039/c4ra05819d日期:——Chemoselective oxidation of allylic alcohols to α,β-unsaturated carbonyl compounds proceeded efficiently using hydrogen peroxide with iron-picolinate catalysts. The in situ generated [Fe(Me-Pic)3] (Me-Pic = 6-methylpicolinate) catalyzed oxidation of the alcohol moiety of primary allylic alcohols while the [Fe(Pic)3] (Pic = picolinate) and [Fe(Me-Pic)2(Pic)] did not show sufficient catalytic activity
表征谱图
-
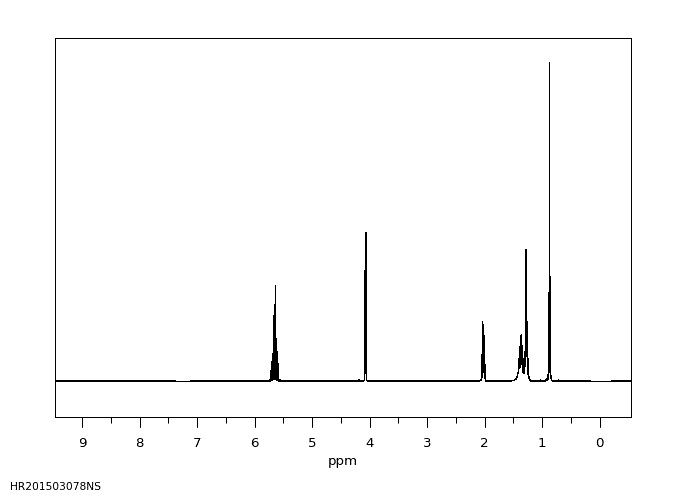
氢谱1HNMR
-
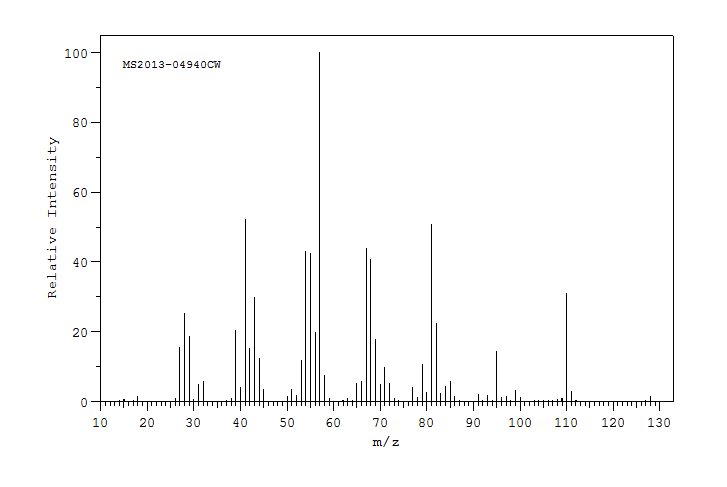
质谱MS
-
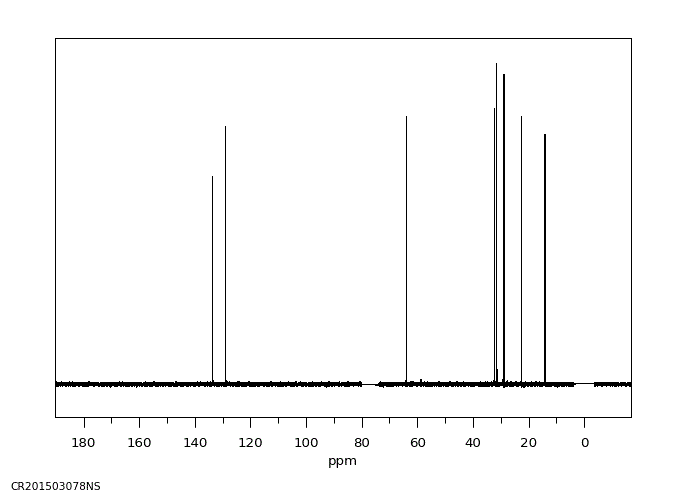
碳谱13CNMR
-
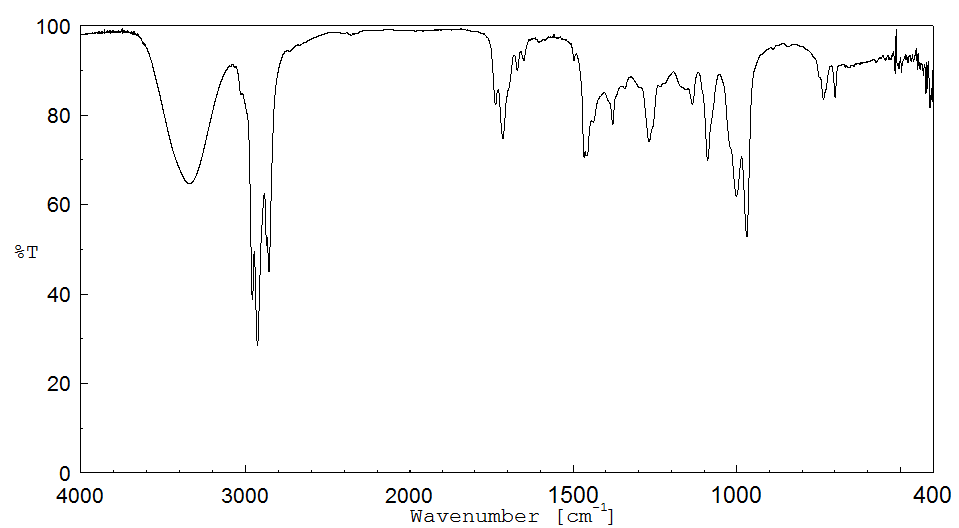
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯