1-氟-1,1,2-三氯乙烷 | 811-95-0
中文名称
1-氟-1,1,2-三氯乙烷
中文别名
——
英文名称
1,1,2-trichloro-1-fluoroethane
英文别名
1-fluoro-1,1,2-trichloroethane;R-131a;1,2,2-trichloro-2-fluoroethane;1,1,2-trichlorofluoroethane;F131a;1,1,2-Trichlor-1-fluor-aethan;1,1,2-trichlorofluorethane;monofluorotrichloroethane;HCFC-131a
CAS
811-95-0
化学式
C2H2Cl3F
mdl
MFCD00042303
分子量
151.395
InChiKey
ZKVMMSGRDBQIOQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-104.7°C
-
沸点:88°C
-
密度:1,492 g/cm3
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:TOXIC, OZONE DEPLETER
-
危险品标志:T
-
危险类别码:R20/21/22,R59
-
危险品运输编号:UN 3082
-
安全说明:S23,S36/37/39
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2-二氯氟乙烷 1,2-dichloro-1-fluoroethane 430-57-9 C2H3Cl2F 116.95
反应信息
-
作为反应物:描述:参考文献:名称:一些氯氟乙烷的热脱卤化氢摘要:DOI:10.1021/ie50497a065
-
作为产物:描述:参考文献:名称:The Chlorination of 1,1-Difluoroethane and 1-Chloro-1,1-difluoroethane1摘要:DOI:10.1021/ja01139a502
-
作为试剂:描述:参考文献:名称:Catalytic process for the preparation of fluorinated halocarbons摘要:描述了一种制备2-氯-1,1,1-三氟乙烷的过程,通过1,2-二氯-1,1-二氟乙烷与氢氟酸反应,在氟化催化剂的存在下。该过程利用了一种速率增强试剂,即三氯乙烯,1-氟-1,2,3-三氯乙烷或具有以下结构的芳香族速率增强试剂,其中R是C1到C6的直链或支链烷基,至少有一个卤素基团,卤素或硝基,R'是C1到C6的直链或支链烷基,至少有一个卤素基团。公开号:US20090018376A1
文献信息
-
Room-temperature catalytic fluorination of C1 and C2 chlorocarbons and chlorohydrocarbons on fluorinated Fe3O4 and Co3O4作者:James ThomsonDOI:10.1039/ft9949003585日期:——A study of the room-temperature reactions of a series of C1 and C2 chlorohydrocarbon and chlorocarbon substrate molecules with fluorinated iron (II, III) oxide and cobalt (II, III) oxide has been conducted. The results show that fluorinated iron(II, III) oxide exhibits an ability to incorporate fluorine into the following substrates in the order: Cl2CCCl2 > H2CCCl2 > CH3CCl3 > CHCl3 > CH2Cl2 > CH2ClCCl3 > CCI4 > CHCl2CHCl2. The fluorinated cobalt(II,III) oxide gave the reactivity series CHCl3 > CCl4 > H2CCCl2 > CHCl2CHCl2 > CH2CI2 > CH3CCl3 > CCl2CCl2 > CH2ClCl3. Reactions of C1 chlorohydrocarbon or chlorocarbon probe molecules with fluorinated Fe3O4 gave predominately C1 chlorofluorohydrocarbon and chlorofluorocarbon products, respectively, whereas fluorinated cobalt(II, III) oxide produced predominately C2 chlorofluorohydrocarbon and chlorofluorocarbons. For fluorinated Co3O4 the distribution of C2 products obtained from C1 chlorohydrocarbon precursor molecules is consistent with the formation of radical intermediates at strong Lewis acid surfaces. C2 chlorohydrocarbons exhibit a fluorine for chlorine (F-for-Cl) exchange reaction through the catalytic dehydrochlorination of the substrate to the alkenic intermediate. The F-for-Cl exchange process was dependent upon the ability of the substrate material to undergo dehydrochlorination; the inability of a substrate to undergo dehydrochlorination results in the fluorination process proceeding through the formation of chlorocarbon or chlorohydrocarbon radical intermediates.对一系列C1和C2氯代烃和氯代碳基底分子室温下与氟化铁(II,III)氧化物和钴(II,III)氧化物的反应进行了研究。结果表明,氟化铁(II,III)氧化物具有将氟引入以下基质的能力,顺序为:Cl2CCCl2 > H2CCCl2 > CH3CCl3 > CHCl3 > CH2Cl2 > CH2ClCCl3 > CCl4 > CHCl2CHCl2。氟化钴(II,III)氧化物给出的反应活性系列为: > > H2CCCl2 > CHCl2CHCl2 > > CH3CCl3 > CCl2CCl2 > CH2ClCl3。C1氯代烃或氯代碳探针分子与氟化Fe3O4反应分别主要生成C1氯氟烃和氯氟碳产物,而氟化钴(II,III)氧化物主要生成C2氯氟烃和氯氟碳。对于氟化Co3O4,从C1氯代烃前体分子获得的C2产物的分布与在强路易斯酸表面形成自由基中间体一致。C2氯代烃通过催化脱氯化氢生成烯烃中间体,进行氟氯(F-for-Cl)交换反应。F-for-Cl交换过程取决于基质材料进行脱氯化氢的能力;基质不能进行脱氯化氢将导致氟化过程通过形成氯代碳或氯代烃自由基中间体进行。
-
Heterogeneous catalyzed synthesis of 1,1,1,2-tetrafluoroethane from 1,1,1,2-tetrachloroethane — thermodynamics and reaction pathways作者:A. Kohne、E. KemnitzDOI:10.1016/0022-1139(95)03320-d日期:1995.11catalyst. The reaction pathways were derived under different conditions. Fluorinated haloalkanes are formed both by dehydrochlorination/hydrofluorination mechanisms as well as a chlorine/fluorine exchange mechanism. Thus, beside fluorinated alkanes considerable amounts of halo-olefins occur in the product mixture. A survey is given of the reaction pathways showing their dependence on the reaction conditions
-
Generation of radical species in surface reactions of chlorohydrocarbons and chlorocarbons with fluorinated gallium(III) oxide or indium(III) oxide作者:J. ThomsonDOI:10.1039/a802365d日期:——catalysts fluorinated gallium(III) oxide and fluorinated indium(III) oxide, respectively. Product analysis shows chlorine-for-fluorine exchange reactions together with the formation of 2-methylpropane and its chlorinated analogues 2-chloromethyl-1,3-dichloropropane and 2-chloromethyl-1,2,3-trichloropropane. Reactivities of the chlorohydrocarbon probe molecules show fluorinated gallium(III) oxide to
-
Synthesis of 1,1,1-trifluoroethane by fluorination of 1-chloro-1, 1-difluoroethane申请人:Atofina公开号:US06339178B1公开(公告)日:2002-01-15The subject of the invention is the manufacture of 1,1,1-trifluoroethane by fluorination or 1-chloro-1,1-difluoroethane with anhydrous hydrofluoric acid. The reaction is carried out in the liquid phase and in the presence of a fluorination catalyst.
-
Boguslavskaya, L. S.; Chuvatkin, N. N.; Panteleeva, I. Yu., Journal of Organic Chemistry USSR (English Translation), 1982, vol. 18, # 10, p. 1832 - 1842作者:Boguslavskaya, L. S.、Chuvatkin, N. N.、Panteleeva, I. Yu.DOI:——日期:——
表征谱图
-
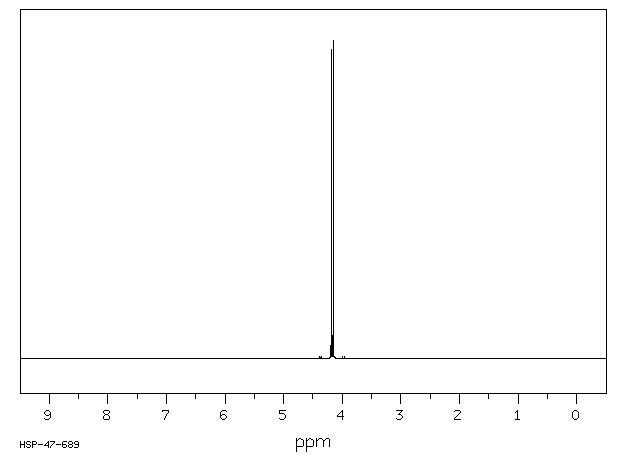
氢谱1HNMR
-
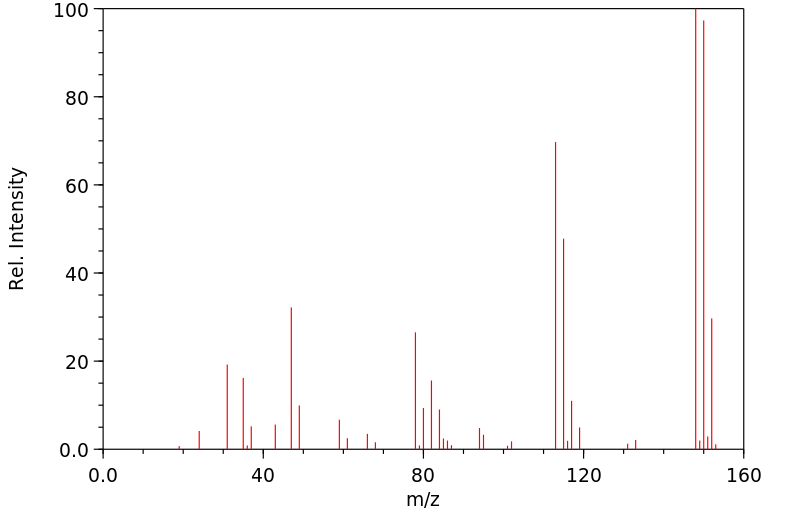
质谱MS
-
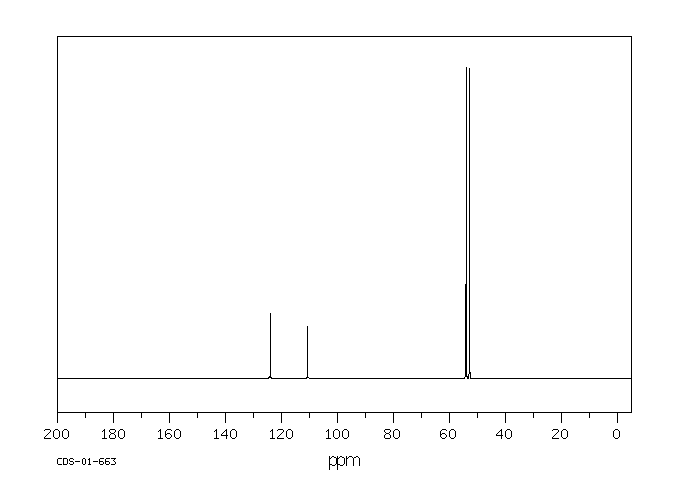
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-氟-环丙胺
顺式-1,1,1,4,4,4-六氟-2-丁烯
顺-1,1,2,2,3,4-六氟环丁烷
酰亚胺基二亚磷酸,甲基-,四(2,2,2-三氟乙基)酯
舒巴坦酸
聚(7-脱氮杂腺嘌呤酸)
癸烷,6-溴-1,1,1,2,2,3,3-七氟-4,4-二(三氟甲基)-
环丙基溴化镁
溴五氟乙烷
氯氟烃-252
氯氟烃-232
氯氟-甲基
氯四氟乙烷
氯二氟乙醛
氯三氟乙烷
氨甲酸,(氟磺酰)-,甲基酯
氢氯氟碳-261
氟甲醇
氟甲基自由基
氟甲基环戊烷
氟甲基环丙烷
氟环辛烷
氟环戊烷
氟环庚烷
氟环十二烷
氟环丁烷
1-溴-1-氯-2,2,2-三氟乙烷
氟氯乙烷
氟化烯丙基
氟化乙亚胺酰基,2-(二氟氨基)-N,2,2-三氟-
氟化丁基
氟乙醛
氟乙烷
氟乙烯醚
正膦胺,N-(2,3,4,5,6-五氯-2,3,4,5,6-五氟亚环己基)-1,1,1,1-四(2,2,3,3-四氟丙氧基)-
桉叶素
替氟烷
恩氟烷
异氟醚
异十八烷酸己酯
己酸,2,5-二氨基-6-羟基-(7CI)
奥替尼啶HCL
壬氟环戊烷
地氟烷
叔丁基氟化物
反式-2-氟环丙胺盐酸盐
反式-2-氟代环戊烷-1-胺盐酸盐
反式-1,2-双(全氟己基)乙烯
反式-1,2-双(全氟-n-丁基)乙烯
反式-1,1,1,2,2,3,3-七氟-4-壬烯