9-苄基芴 | 1572-46-9
中文名称
9-苄基芴
中文别名
——
英文名称
9-benzylfluorene
英文别名
9-benzyl-9H-fluorene
CAS
1572-46-9
化学式
C20H16
mdl
——
分子量
256.347
InChiKey
ZBQLAOVNDBNMFI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.3
-
重原子数:20
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
安全说明:S22,S24/25
-
海关编码:2902909090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— fluorene-9-carbaldehyde 20615-64-9 C14H10O 194.233 —— 9-benzyl-9-fluorenol 36322-03-9 C20H16O 272.346 —— 9-Benzyl-fluoren-9-carbonsaeure 4709-69-7 C21H16O2 300.357 芴 9H-fluorene 86-73-7 C13H10 166.222 9-芴酮 9-fluorenone 486-25-9 C13H8O 180.206 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 9-benzyl-9-methylfluorene 61076-90-2 C21H18 270.374 —— 9,9-Dibenzyl-fluoren 4709-64-2 C27H22 346.472 —— 9-benzyl-9-fluorenol 36322-03-9 C20H16O 272.346 —— 9-butyl-9-benzylfluorene 88223-32-9 C24H24 312.455 —— 1-[4-(9-Benzylfluoren-9-yl)but-2-ynyl]azepane —— C30H31N 405.583 芴 9H-fluorene 86-73-7 C13H10 166.222 9-芴酮 9-fluorenone 486-25-9 C13H8O 180.206
反应信息
-
作为反应物:参考文献:名称:通过卤化钴自由基偶联合成二芴摘要:原位生成的氟化锂盐与氯化钴的反应导致形成相应的双芴。可以偶联的芴的范围包括那些含有适于聚合的取代基用于聚(二芴)合成的取代基和用于制备双金属过渡金属配合物的烷基取代的芴。基于使用自由基阱的实验,反应机理涉及还原钴和随后的芴基自由基偶联。总的来说,这项研究证明了使用容易获得且价格便宜的偶联剂制备受阻第三级和第四级CC键的简单有效的方法。DOI:10.1016/j.tetlet.2011.03.127
-
作为产物:描述:参考文献:名称:锰催化的季铵盐的合成:在催化过氧化和重排反应中的应用。摘要:使用Mn-2,2'-联吡啶催化剂通过自由基-自由基交叉偶合,实现了9-取代芴的高效,选择性和直接CH-H过氧化。而且,该方法有效地促进了空间受阻的各种取代的亚芳基-9 H-芴/芳基吲哚-2-酮衍生物的邻位双过氧化,从而提供了高取代度的双过氧化物,其对通常形成环戊二烯酮的C═C键的氧化裂解具有高选择性。醛。此外,一种合成(Z)-6-亚苄基-6 H-苯并[ c的新方法通过这些过氧化物的酸催化的骨架重排已经获得了] 1,1-二烯。与O-O键断裂不同,这是首次描述了使用Pd催化剂和H 2在过氧化物中进行还原性C-O键断裂,这使得可逆反应可提供完全脱氧的产物。已经提出了过氧化,分子重排和脱过氧化的详细机理,并提供了初步的实验证据。DOI:10.1021/acs.joc.0c00837
-
作为试剂:描述:参考文献:名称:Carbon acidity. 77. Ion pair carbon acidities of some silanes in tetrahydrofuran摘要:The relative solvent-separated ion pair (SSIP) lithium acidity (PK(Li)/THF) and contact ion pair (CIP) cesium acidity (pK(Cs/THF)) were obtained for 9-fluorenyltrimethylsilane (1) (21.3, 21.6, respectively) and 9-fluorenyl-tert-butyldimethylsilane (2) (20.3, 20.6, respectively) in THF. Values for pK(Cs/THF) were determined at 25-degrees-C for (p-biphenylylmethyl)-tert-butyldimethylsilane (3), 35.4, benzyltrimethylsilane (4), 37.5, alpha,alpha-bis(trimethylsilyl)toluene (5), 34.1, 2-(trimethylsilyl)-1,3-dithiane (6), 33.5, (trimethylsilyl)acetonitrile (7), 28.8, and tris(trimethylsilyl)methane (8), 36.8. Some thermodynamic parameters were determined by measurements at other temperatures, and some ionic acidities (pK(FI)) were determined by conductivity studies. Carbanion stabilization by these silyl substituents varies from about 1 to over 3 pK units in different systems. 9,9-Bis(trimethylsilyl)fluorene (9) was found to undergo silyl transfer on treatment with various carbanions, but this reaction is slower than proton transfer.DOI:10.1021/jo00059a031
文献信息
-
Nickel-catalyzed synthesis of 9-monoalkylated fluorenes from 9-fluorenone hydrazone and alcohols作者:Jiang-Tao Fan、Xin-Heng Fan、Yong-Jie Chen、Cai-Yan Gao、Lian-Ming YangDOI:10.1080/00397911.2019.1647438日期:——Abstract A practical protocol was disclosed for the nickel-catalyzed C-alkylation of 9-fluorenone hydrazone with alcohols using t-BuOK as the base, and 9-monoalkylated fluorene derivatives were obtained in good yields under the benign conditions. GRAPHICAL ABSTRACT
-
ω-Phenylalkyl-substituted zirconocene dichloride complexes as catalyst precursors for homogeneous ethylene polymerization作者:Erik H Licht、Helmut G Alt、M.Manzurul KarimDOI:10.1016/s0022-328x(00)00009-7日期:2000.4The reaction of ω-phenyl-1-bromoalkanes with cyclopentadienyl sodium, indenyl lithium or fluorenyl lithium forms ω-phenylalkyl-substituted ligand precursors in high yields. The corresponding anions react with zirconium tetrachloride to give ω-phenylalkyl-substituted zirconocene dichloride complexes. After activation with methylaluminoxane, these complexes are highly active catalysts for homogeneous
-
Hydrogenation reactions with hydridocobalt Tetracarbonyl作者:Theodore E. Nalesnik、John H. Freudenberger、Milton OrchinDOI:10.1016/s0022-328x(00)89054-3日期:1981.12The tetrasubstituted ethylene, bifluorenylidene, reacts very rapidly (4.06 X 10−2 1 mol−1 sec−1 at 0°C) with HCo(CO)4 to give bifluorenyl. α-Phenylacrylonitrile (atroponitrile) reacts even more rapidly under the same conditions (6.0 l mol−sec−1). Other highly substituted ethylenes react very slowly with HCo(CO)4, indicating considerable steric effects. The data are consistent with radical type intermediates
-
Microwave-assisted synthesis of substituted phenanthrenes, anthracenes, acenaphthenes, and fluorenes作者:Shiuh-Chuan Chan、Jing-Pei Jang、Yie-Jia CherngDOI:10.1016/j.tet.2009.01.029日期:2009.3Rapid coupling reactions of polycyclic aromatic halides with various N-, S-, and Se-nucleophiles under focused microwave irradiation are described. Using this method, the desired products are obtained with good to excellent yields in a short reaction time.描述了在聚焦微波辐射下多环芳族卤化物与各种N-,S-和Se-亲核试剂的快速偶联反应。使用这种方法,可以在短的反应时间内以良好或极好的收率获得所需的产物。
-
Study on the Reactivity of Diarylmethane Derivatives in Supercritical Alcohols Media: Reduction of Diarylmethanols and Diaryl Ketones to Diarylmethanes Using Supercritical 2-Propanol作者:Bunpei Hatano、Daisuke Kubo、Hideyuki TagayaDOI:10.1248/cpb.54.1304日期:——We found that diarylmethanols and diaryl ketones were smoothly reduced to the corresponding diarylalkanes using supercritical 2-propanol in good yields. Furthermore, we determined the specific reaction of fluorene using supercritical methanol at high temperature.
表征谱图
-
氢谱1HNMR
-
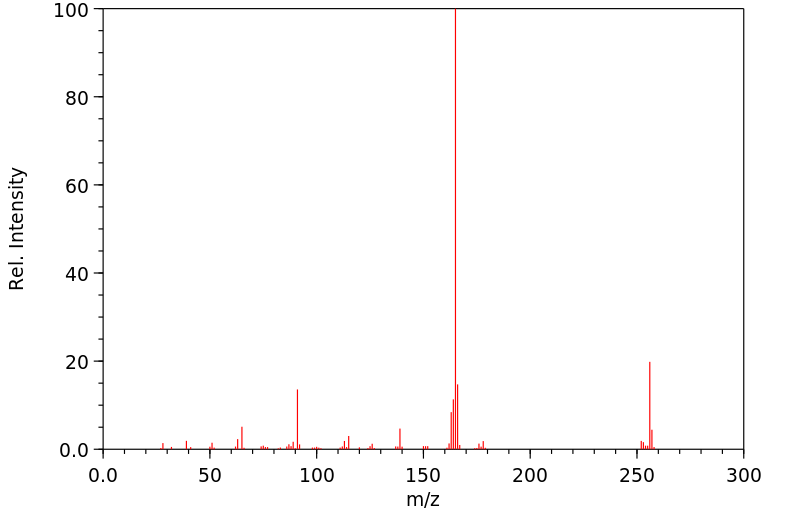
质谱MS
-
碳谱13CNMR
-
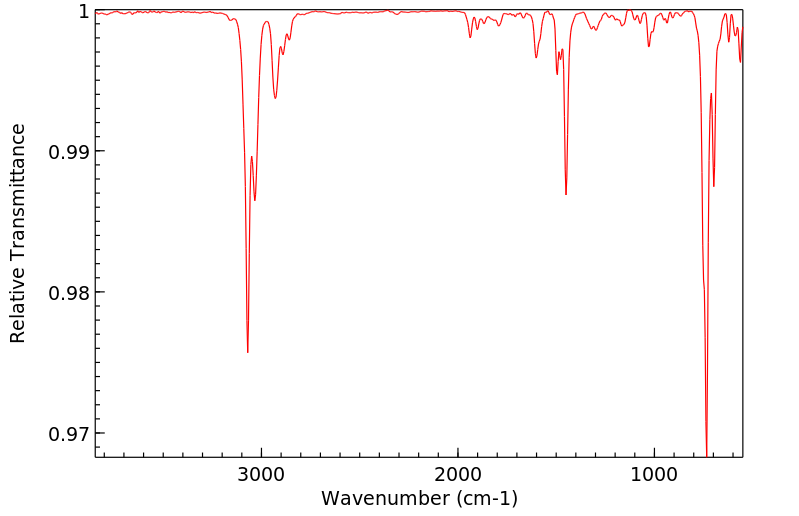
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-2-N-Fmoc-氨基甲基吡咯烷盐酸盐
(2S,4S)-Fmoc-4-三氟甲基吡咯烷-2-羧酸
黎芦碱
鳥胺酸
魏因勒卜链接剂
雷迪帕韦二丙酮合物
雷迪帕韦中间体6
雷迪帕韦
雷迪帕维中间体
雷迪帕维中间体
雷尼托林
锰(2+)二{[乙酰基(9H-芴-2-基)氨基]氧烷负离子}
醋酸丁酸纤维素
达托霉素杂质
赖氨酸杂质4
试剂9,9-Dioctyl-9H-fluoren-2-amine
螺[环戊烷-1,9'-芴]
螺[环庚烷-1,9'-芴]
螺[环己烷-1,9'-芴]
螺[3.3]庚烷-2,6-二-(2',2'',7',7''-四碘螺芴)
螺-(金刚烷-2,9'-芴)
螺(环己烷-1,9'-芴)-3-酮
藜芦托素
荧蒽 反式-2,3-二氢二醇
草甘膦-FMOC
英地卡胺
苯芴醇杂质A
苯甲酸-(芴-9-基-苯基-甲基酯)
苯甲酸-(9-苯基-芴-9-基酯)
苯并[b]芴铯盐
苯并[a]芴酮
苯基芴胺
苯基(9-苯基-9-芴基)甲醇
苯(甲)醛,9H-芴-9-亚基腙
苯(甲)醛,4-羟基-3-甲氧基-,(3-甲基-9H-茚并[2,1-c]吡啶-9-亚基)腙
芴甲氧羰酰胺
芴甲氧羰酰基高苯丙氨酸
芴甲氧羰酰基肌氨酸
芴甲氧羰酰基环己基甘氨酸
芴甲氧羰酰基正亮氨酸
芴甲氧羰酰基D-环己基甘氨酸
芴甲氧羰酰基D-Β环己基丙氨酸
芴甲氧羰酰基-O-三苯甲基丝氨酸
芴甲氧羰酰基-D-正亮氨酸
芴甲氧羰酰基-6-氨基己酸
芴甲氧羰基-高丝氨酸内酯
芴甲氧羰基-缬氨酸-1-13C
芴甲氧羰基-叔丁基二甲基硅-D-丝氨酸
芴甲氧羰基-beta-赖氨酰酸(叔丁氧羰基)
芴甲氧羰基-S-叔丁基-L-半胱氨酸五氟苯基脂