4,4'-二甲苯亚砜 | 1774-35-2
中文名称
4,4'-二甲苯亚砜
中文别名
对甲苯基亚砜;4,4'-二甲基二苯基亚砜;4,4’-二甲苯亚砜;4,4"-二甲苯亚砜
英文名称
di(p-tolyl) sulfoxide
英文别名
p-tolyl sulfoxide;1-methyl-4-(4-methylphenyl)sulfinylbenzene
CAS
1774-35-2
化学式
C14H14OS
mdl
MFCD00008546
分子量
230.331
InChiKey
MJWNJEJCQHNDNM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:94-96 °C (lit.)
-
沸点:378.3±31.0 °C(Predicted)
-
密度:1.19±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:36.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
WGK Germany:3
-
RTECS号:XT4810000
-
海关编码:2930909090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温
SDS
p-Tolyl Sulfoxide Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: p-Tolyl Sulfoxide
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: p-Tolyl Sulfoxide
Percent: >98.0%(GC)
CAS Number: 1774-35-2
Synonyms: Di-p-tolyl Sulfoxide
Chemical Formula: C14H14OS
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
p-Tolyl Sulfoxide
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Almost white
Odour: No data available
pH: No data available
Melting point/freezing point:94°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
p-Tolyl Sulfoxide
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
ipr-mus LD50:600 mg/kg
Acute Toxicity:
ivn-mus LD50:180 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
XT4810000
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
p-Tolyl Sulfoxide
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: p-Tolyl Sulfoxide
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: p-Tolyl Sulfoxide
Percent: >98.0%(GC)
CAS Number: 1774-35-2
Synonyms: Di-p-tolyl Sulfoxide
Chemical Formula: C14H14OS
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
p-Tolyl Sulfoxide
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Almost white
Odour: No data available
pH: No data available
Melting point/freezing point:94°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
p-Tolyl Sulfoxide
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
ipr-mus LD50:600 mg/kg
Acute Toxicity:
ivn-mus LD50:180 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
XT4810000
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
p-Tolyl Sulfoxide
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途
4,4'-二甲苯亚砜是一种亚砜化合物。已上市的代表性药物如埃索美拉唑(A.Aktiebolag, US5877192A, 1998)用于治疗肠胃疾病,以及莫达非尼(L.L.Lafon, US4927855A, 1990),临床用于治疗嗜睡症和厌食症。亚砜骨架结构的多样性及其显著的生物学功能吸引了有机合成化学家和药学家的关注。
此外,亚砜类化合物还具有重要的生物活性,通过对亚砜类化合物进行结构修饰可以提高其药效活性,如抗溃疡、抗病毒、抗HIV-1、抗肿瘤等。在农药领域,亚砜类化合物因其用量少、低污染的优点而作为杀菌剂和除草剂使用。由于含有半极性基团,亚砜是一种软碱类中性萃取剂,对软酸类的贵金属有特殊的亲和力,因此其对贵金属的萃取率高且选择性好。同时,亚砜是许多天然产物及药物中间体的重要组成部分。因此,发展高效快捷合成亚砜的方法具有重要意义,并成为热点研究领域。
制备在15 mL反应管中依次加入对甲基苯偶氮甲基砜(0.6 mmol)、对甲基苯硫酚(0.2 mmol)、乙腈(1 mL)和水(1 mL),混合均匀后,在3 W蓝色LED灯照射下,于25°C搅拌反应16小时。用TLC检测至反应完全后,加入乙酸乙酯萃取三次,每次使用3 mL,合并萃取液并经过无水硫酸钠干燥。将萃取液在真空度为0.08 Mpa的条件下减压浓缩至无溶剂,得到粗产物。然后用体积比为5:1的石油醚和乙酸乙酯混合洗脱剂进行冲洗,并通过硅胶柱快速层析,最终得到4,4'-二甲苯亚砜,收率为70%,为黄色油状固体32.2 mg。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (R)-(+)-phenyl p-tolyl sulfoxide 16491-20-6 C13H12OS 216.304 2-甲基二苯并[b,d]噻吩5-氧化物 1-benzenesulfinyl-4-methyl-benzene 77096-69-6 C13H12OS 216.304 4,4’-二甲基二苯硫醚 di-(p-tolyl)sulfane 620-94-0 C14H14S 214.331 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲基二苯并[b,d]噻吩5-氧化物 1-benzenesulfinyl-4-methyl-benzene 77096-69-6 C13H12OS 216.304 二对甲苯酰硫 Di-p-tolyl sulfone 599-66-6 C14H14O2S 246.33 二苯基亚砜 1,1'-sulfinylbisbenzene 945-51-7 C12H10OS 202.277 4,4’-二甲基二苯硫醚 di-(p-tolyl)sulfane 620-94-0 C14H14S 214.331 苯,1-甲基-3-[(4-甲基苯基)硫代]- 3-methylphenyl 4-methylphenyl sulfide 107770-92-3 C14H14S 214.331
反应信息
-
作为反应物:描述:4,4'-二甲苯亚砜 在 N-溴代丁二酰亚胺(NBS) 、 偶氮二异丁腈 作用下, 以 四氯化碳 为溶剂, 反应 12.0h, 以78.1%的产率得到4-(bromomethyl)-1-[(4-methylphenyl)sulfinyl]benzene参考文献:名称:Arylthiobenzylpiperidine derivatives摘要:这项发明涉及芳基硫苄哌啶衍生物,其是MCH1受体的配体。该发明提供了一种包括该发明化合物的治疗有效量和药用载体的药物组合物。该发明还提供了一种通过混合该发明化合物的治疗有效量和药用载体制备的药物组合物。该发明还提供了一种制备药物组合物的方法,包括组合该发明化合物的治疗有效量和药用载体。该发明还提供了一种治疗患有抑郁症和/或焦虑症的受试者的方法,包括向受试者施用该发明化合物的剂量。该发明还提供了一种治疗患有肥胖症的受试者的方法,包括向受试者施用该发明化合物的剂量。公开号:US20060079683A1
-
作为产物:描述:对甲苯基溴化镁 在 1,1'-binaphthyl-2,2'-diyl sulfite 作用下, 生成 4,4'-二甲苯亚砜参考文献:名称:外消旋和旋光 1,1'-binaphthyl-2,2'-diyl亚硫酸盐:合成、晶体结构和与选定亲核试剂的开环反应摘要:报道了衍生自 BINOL 的外消旋和 (R)-(+)-对映异构体的亚硫酸盐的制备。介绍了旋光、左旋亚硫酸盐异构体的晶体结构及其通过与选定亲核试剂的亲核取代反应引起的开环。© 2011 Wiley Periodicals, Inc. 杂原子化学 22:562–570, 2011; 在 wileyonlinelibrary.com 上在线查看这篇文章。DOI 10.1002/hc.20722DOI:10.1002/hc.20722
-
作为试剂:描述:乙烯氧基三甲基硅烷 、 methyl (3aR,4R,6S,7aS)-3-acetyl-6-(4-methylphenyl)sulfanyl-2-oxo-4-[(1S,2R)-1,2,3-triacetyloxypropyl]-3a,4,7,7a-tetrahydropyrano[3,4-d][1,3]oxazole-6-carboxylate 在 4,4'-二甲苯亚砜 、 三氟甲磺酸酐 作用下, 以 二氯甲烷 为溶剂, 反应 4.75h, 以43%的产率得到methyl (2-C-(2-oxoethyl)-5-N-acetamido-7,8,9-tri-O-acetyl-5-N,4-O-carbonyl-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranitol)onate参考文献:名称:Highly efficient α-C-sialylation promoted by (p-Tol)2SO/Tf2O with N-acetyl-5-N,4-O-oxazolidione protected thiosialoside as donor摘要:基于(p-Tol)2SO/Tf2O预活化策略,通过N-乙酰基-5-N,4-O-恶唑烷酮保护的硫代唾液酸与各种三甲基硅基烯醇醚及烯丙基三甲基硅烷的偶联反应,实现了α-唾液酸型C-糖苷的实用、简便及高产率合成。对于具有大亲核性值(N = 4.4–9.0)的强π-亲核试剂,无论是硅醚烯醇、硅醚乙缩醛还是烯丙基三甲基硅烷作为亲电C-唾液酸化试剂,均能获得高产率和极佳的α-选择性。DOI:10.1039/c3ob40876k
文献信息
-
Catalyst-free visible-light-initiated oxidative coupling of aryldiazo sulfones with thiols leading to unsymmetrical sulfoxides in air作者:Qishun Liu、Leilei Wang、Huilan Yue、Jiang-Sheng Li、Zidan Luo、Wei WeiDOI:10.1039/c9gc00222g日期:——A facile and efficient visible-light-driven method has been developed to construct sulfoxides via oxidative coupling of aryldiazo sulfones with thiols using the O2 in air as the oxidant. This reaction could be performed at room temperature under catalyst- and additive-free conditions. The present methodology offers a mild and environmentally benign approach to obtain a library of sulfoxides in good已经开发了一种简便有效的可见光驱动方法,通过使用空气中的O 2作为氧化剂,通过芳基重氮砜与硫醇的氧化偶联来构建亚砜。该反应可以在室温下在无催化剂和无添加剂的条件下进行。本方法学提供了温和且对环境无害的方法,以良好的收率和有利的官能团耐受性获得了亚砜的文库。
-
Chlorotrimethylsilane−Nitrate Salts as Oxidants: Direct Oxidative Conversion of Thiols and Disulfides to Sulfonyl Chlorides作者:G. K. Surya Prakash、Thomas Mathew、Chiradeep Panja、George A. OlahDOI:10.1021/jo070907g日期:2007.7.1nitrate salt and chlorotrimethylsilane is found to be a mild and efficient reagent for the direct oxidative conversion of thiols (1) and disulfides (2) to the corresponding sulfonyl chlorides (3) in excellent yields through oxidative chlorination. Sulfides and sulfoxides were also found to undergo oxidation to sulfones under similar reaction conditions. In most cases these reactions are highly selective
-
Synthesis of <i>o</i>-Aryloxy Triarylsulfonium Salts via Aryne Insertion into Diaryl Sulfoxides作者:Xiaojin Li、Yan Sun、Xin Huang、Lei Zhang、Lichun Kong、Bo PengDOI:10.1021/acs.orglett.6b03840日期:2017.2.17The aryne insertion into “S═O” bond has been validated recently. This technology is elusively applied to the synthesis of thioethers. In contrast to the reported cases, the reaction described furnished o-aryloxy triarylsulfonium salts, in lieu of thioethers, in good to excellent yields. The reaction is also featured by its exquisite regioselectivity, broad substrate scope, and mild conditions (25 °C)
-
Triazole oxime derivatives having antimycotic acitivity申请人:MOCHIDA PHARMACEUTICAL CO., LTD.公开号:EP0670315A1公开(公告)日:1995-09-06Triazole oxime derivatives represented by the formula (I): (where Ar is a phenyl group substituted by 1 or 2 halogen atoms; R¹ and R² are typically such that, when taken together with the adjacent carbon atom, they form a cyclopropylidene group; R³ is a straight-chained, branched or cyclic alkyl group having 1 - 4 carbon atoms; R⁴ is typically an optionally substituted straight-chained or branched alkyl group having 1 - 4 carbon atoms; and the wavy line represents either an E- or Z-type bond) or salts thereof. The triazole derivatives exhibit a marked therapeutic effects not only in in vitro experiments but also in in vivo experiments using laboratory animal models such as Aspergillus infected mice. The derivatives are also safe to use. Therefore, they are extremely useful as therapeutics for various superficial dermatomycoses, deep dermatomycoses and deep mycoses (mycoses in internal organs).Triazole oxime 衍生物的化学式(I)表示为: (其中 Ar 是一个被 1 或 2 个卤素原子取代的苯基;R¹ 和 R² 通常是这样的,当与相邻的碳原子一起时,它们形成一个环丙烯基团;R³ 是一个直链、支链或环状的含有 1 - 4 个碳原子的烷基团;R⁴ 通常是一个可选择取代的直链或支链烷基团,含有 1 - 4 个碳原子;波浪线代表 E- 或 Z- 类型键)或其盐。这些三唑衍生物不仅在体外实验中表现出显著的治疗效果,而且在使用实验动物模型(如感染曲霉的小鼠)进行体内实验时也表现出显著的治疗效果。这些衍生物也是安全的。因此,它们在治疗各种浅表真菌病、深部真菌病和深部真菌病(内脏器官真菌病)方面非常有用。
-
一种亚砜化合物的制备方法
表征谱图
-
氢谱1HNMR
-
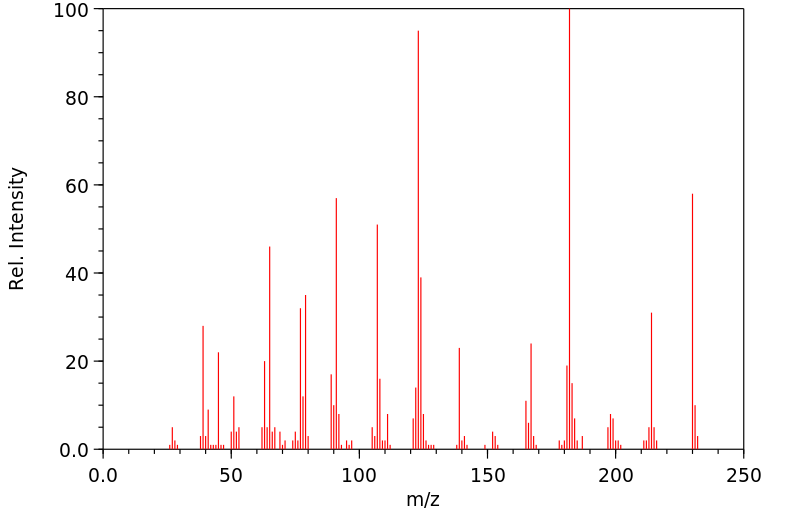
质谱MS
-
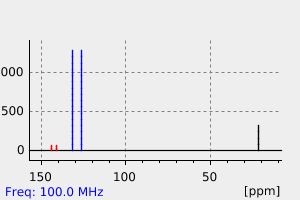
碳谱13CNMR
-
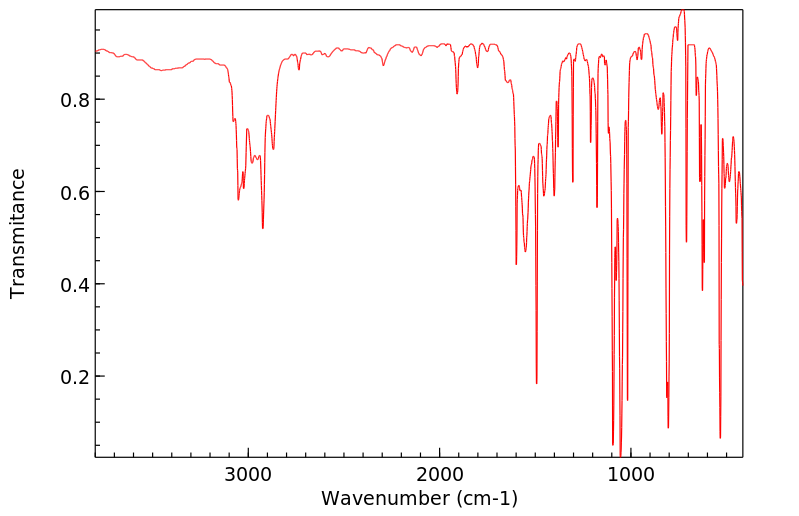
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫