2-氯丙腈 | 1617-17-0
中文名称
2-氯丙腈
中文别名
——
英文名称
2-chlorpropionitrile
英文别名
2-chloropropionitrile;2-chloropropanenitrile;α-chloropropionitrile;beta-chloropropionitrile
CAS
1617-17-0;70886-58-7
化学式
C3H4ClN
mdl
MFCD00001859
分子量
89.5245
InChiKey
JNAYPRPPXRWGQO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:54-55 °C
-
沸点:120-122 °C (lit.)
-
密度:1.012 g/mL at 25 °C (lit.)
-
闪点:92 °F
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:5
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:23.8
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:3.2
-
危险品标志:T
-
安全说明:S16,S26,S27,S36/37/39,S45
-
危险类别码:R10
-
WGK Germany:3
-
海关编码:2926909090
-
包装等级:III
-
危险类别:3.2
-
危险品运输编号:UN 3275 6.1/PG 2
SDS
反应信息
-
作为反应物:参考文献:名称:US2385549摘要:公开号:
-
作为产物:描述:参考文献:名称:镍催化的杂芳基碘化物和 α-氯腈之间的不对称还原交叉偶联摘要:已开发出 Ni 催化的杂芳基碘化物和 α-氯腈的不对称还原交叉偶联。该方法从简单的有机卤化物构建块中提供对映体富集的 α,α-二取代腈。该反应耐受多种杂环偶联物,包括吡啶、嘧啶、喹啉、噻吩和哌啶。该反应在温和的室温条件下进行,不需要预生成有机金属亲核试剂。DOI:10.1021/jacs.5b06466
文献信息
-
Reinvestigation of the Reaction of Trichloroacetyl Chloride and Acrylonitrile in the Preparation of 3,5,6-Trichloropyridin-2-ol作者:H. Fakhraian、A. Moghimi、A. Bazaz、H. Hadj-Ghanbary、M. SadeghiDOI:10.1021/op025610t日期:2003.5.1The synthesis of 3,5,6-trichloropyridin-2-ol via the CuCl-catalyzed reaction of trichloroacetyl chloride and acrylonitrile under both pressure and atmospheric conditions and the hydrolysis of the reaction mixture were reinvestigated. The products and byproducts formed in each case, before the hydrolysis step, were characterized, and the factors causing their formation are discussed. It was found that
-
A Photochemical Organocatalytic Strategy for the α‐Alkylation of Ketones by using Radicals作者:Davide Spinnato、Bertrand Schweitzer‐Chaput、Giulio Goti、Maksim Ošeka、Paolo MelchiorreDOI:10.1002/anie.201915814日期:2020.6.8Reported herein is a visible‐light‐mediated radical approach to the α‐alkylation of ketones. This method exploits the ability of a nucleophilic organocatalyst to generate radicals upon SN2‐based activation of alkyl halides and blue light irradiation. The resulting open‐shell intermediates are then intercepted by weakly nucleophilic silyl enol ethers, which would be unable to directly attack the alkyl
-
Vicarious nucleophilic substitution of hydrogen in nitroderivatives of five-membered heteroaromatic compounds作者:Mieczyslaw Makosza、Ewa KwastDOI:10.1016/0040-4020(95)00445-e日期:1995.7Nitro derivatives of thiophene, furan and pyrrole react with carbanions containing leaving groups giving products of replacement of hydrogen with functionalized alkyl substituents. Many specific features of this reaction are discussed.
-
Anti-emetic quinuclidinyl benzamides申请人:Bristol-Myers Company公开号:US04820715A1公开(公告)日:1989-04-11Novel substituted benzamides of the formula ##STR1## wherein R.sup.1, R.sup.2, R.sup.3, R.sup.4, R.sup.5 and A are as defined herein are useful in the treatment of emesis, and particularly chemotherapy-induced emesis in cancer patients. Some of the compounds are also useful in disorders relating to impaired gastric motility.式中R1、R2、R3、R4、R5和A的定义如本文所述,新型取代苯甲酰胺用于治疗呕吐,特别是癌症患者的化疗引起的呕吐。其中一些化合物也用于治疗与胃动力减弱相关的疾病。
-
NAPHTHYRIDINES AS INHIBITORS OF HPK1申请人:Genentech, Inc.公开号:US20180282328A1公开(公告)日:2018-10-04Naphthyridine compounds and their use as inhibitors of HPK1 are described. The compounds are useful in treating HPK1-dependent disorders and enhancing an immune response. Also described are methods of inhibiting HPK1, methods of treating HPK1-dependent disorders, methods for enhancing an immune response, and methods for preparing the naphthyridine compounds.
表征谱图
-
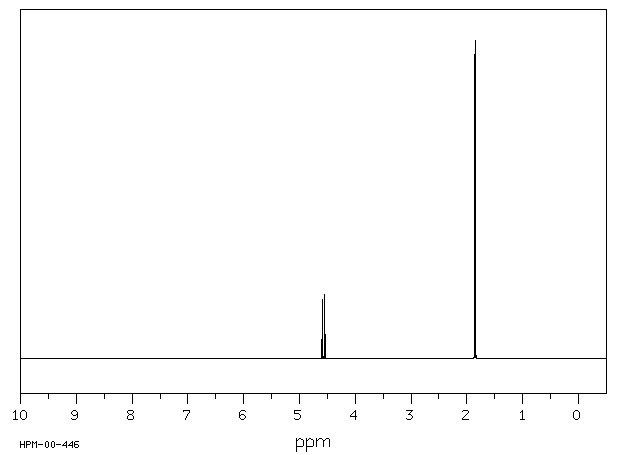
氢谱1HNMR
-
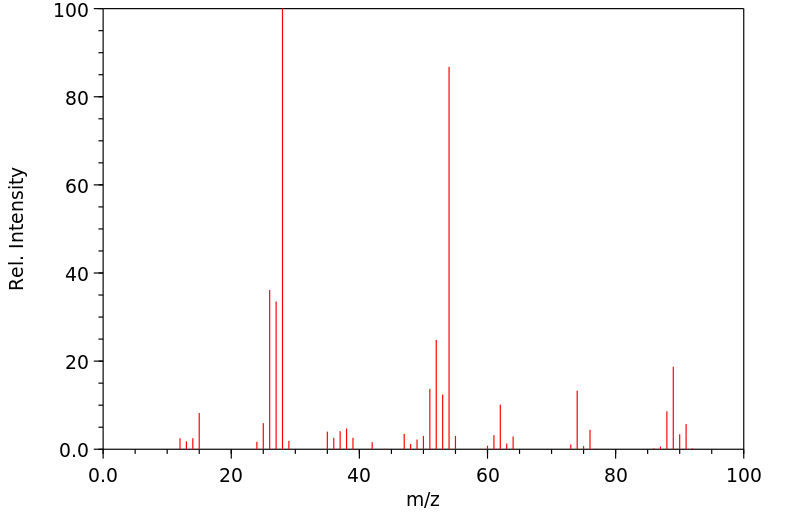
质谱MS
-
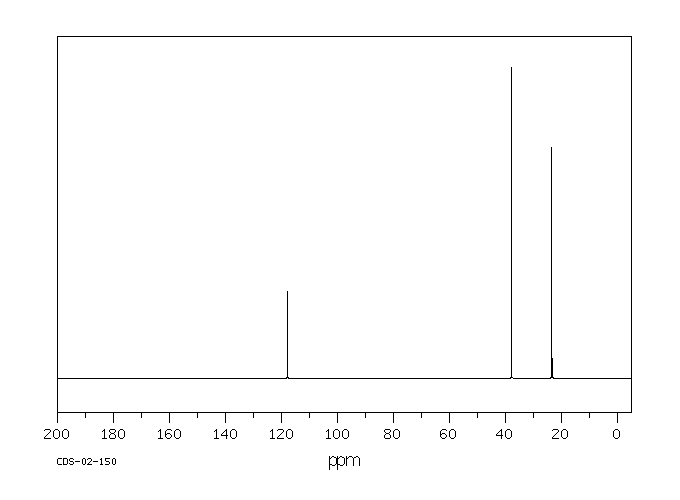
碳谱13CNMR
-
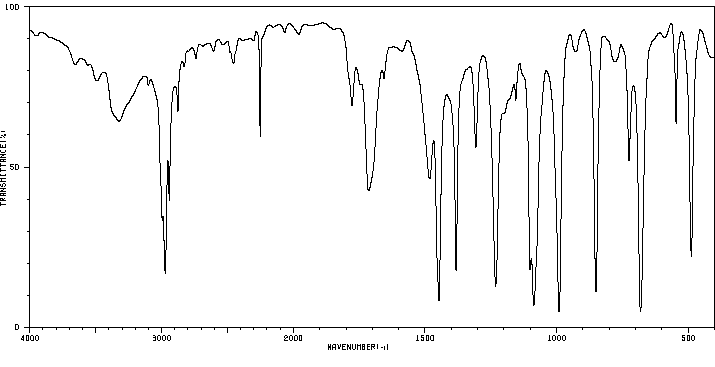
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷