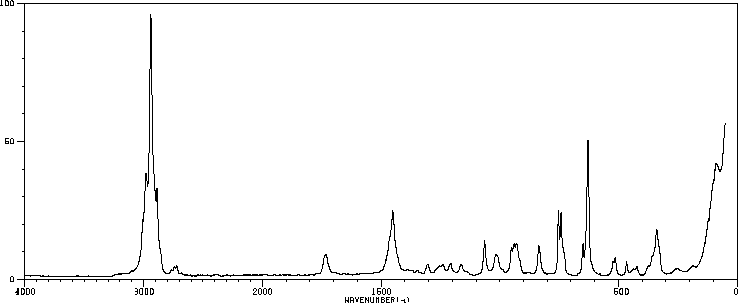
Version 1.0
Regulation (EC) No 1907/2006
1 - Product and Company Information
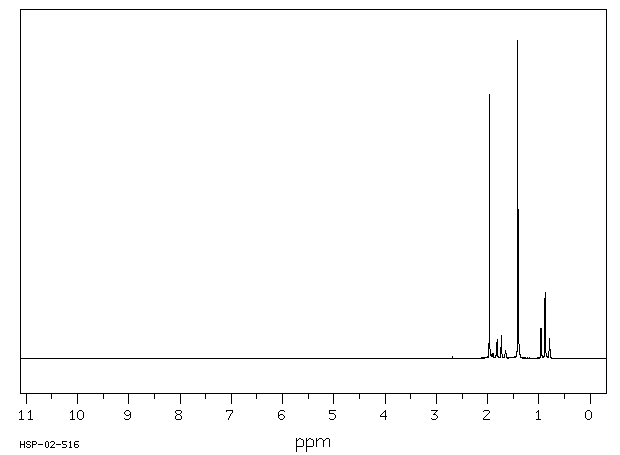
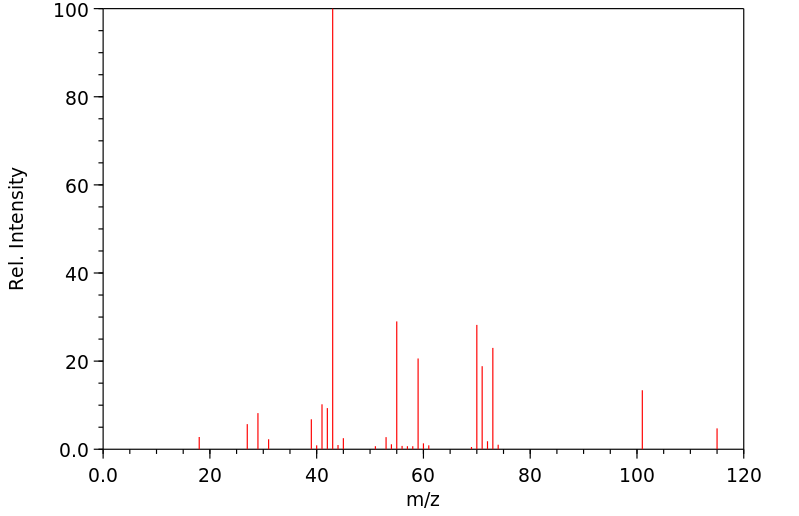
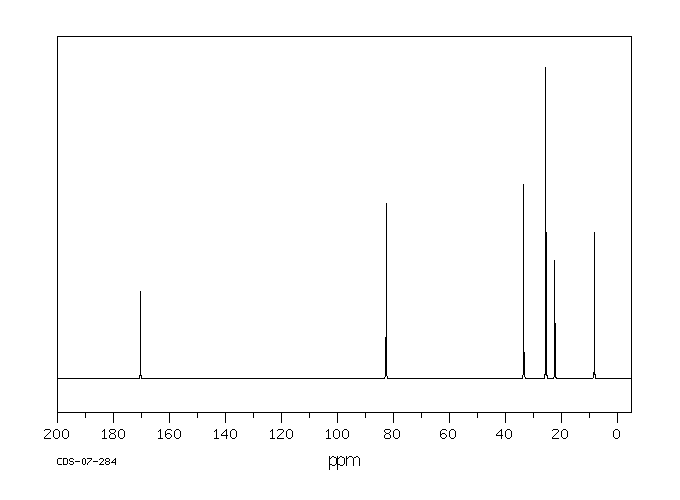
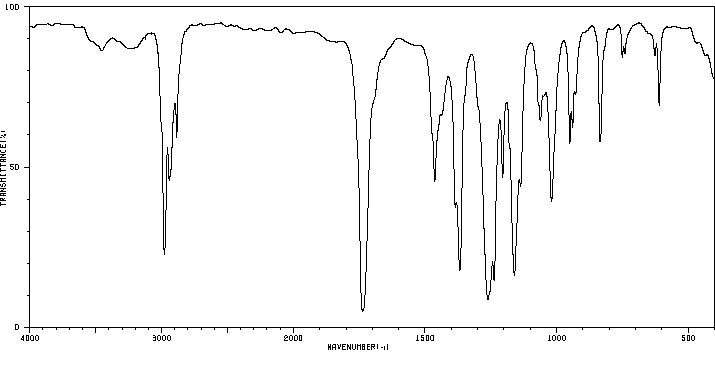
Product Name TERT-AMYL ACETATE - 50 MG
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
Flammable.
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
TERT-AMYL ACETATE 625-16-1 None None
Molecular Weight 130,1800 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician immediately.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
ALDRICH www.molbase.com
Suitable: For small (incipient) fires, use media such as
"alcohol" foam, dry chemical, or carbon dioxide. For large
fires, apply water from as far as possible. Use very large
quantities (flooding) of water applied as a mist or spray; solid
streams of water may be ineffective. Cool all affected
containers with flooding quantities of water.
SPECIAL RISKS
Specific Hazard(s): Flammable liquid. Emits toxic fumes under
fire conditions.
Explosion Hazards: Vapor may travel considerable distance to
source of ignition and flash back. Container explosion may occur
under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PERSONAL PRECAUTION PROCEDURES TO BE FOLLOWED IN CASE OF LEAK OR SPILL
Evacuate area. Shut off all sources of ignition. Use nonsparking
tools.
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear self-contained breathing apparatus, rubber boots, and heavy
rubber gloves.
METHODS FOR CLEANING UP
Cover with dry-lime, sand, or soda ash. Place in covered
containers using non-sparking tools and transport outdoors.
Ventilate area and wash spill site after material pickup is
complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid contact with eyes, skin, and
clothing. Do not breathe vapor. Avoid prolonged or repeated
exposure.
STORAGE
Conditions of Storage: Keep container closed. Keep away from
heat, sparks, and open flame.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Use nonsparking tools. Mechanical
exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Where risk assessment shows air-purifying respirators
are appropriate use a full-face respirator with multi-purpose
combination (US) or type ABEK (EN 14387) respirator cartridges as
ALDRICH www.molbase.com
a backup to engineering controls. If the respirator is the sole
means of protection, use a full-face supplied air respirator.
Hand Protection: Compatible chemical-resistant gloves.
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
Appearance Physical State: Liquid
Property Value At Temperature or Pressure
pH N/A
BP/BP Range N/A
MP/MP Range N/A
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient Log Kow: 2,000
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Skin Absorption: May be harmful if absorbed through the skin.
Eye Contact: May cause eye irritation.
Inhalation: Material may be irritating to mucous membranes and
upper respiratory tract. May be harmful if inhaled.
Ingestion: May be harmful if swallowed.
12 - Ecological Information
No data available.
ALDRICH www.molbase.com
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Contact a licensed professional waste disposal service to dispose
of this material. Burn in a chemical incinerator equipped with an
afterburner and scrubber but exert extra care in igniting as this
material is highly flammable. Observe all federal, state, and
local environmental regulations.
14 - Transport Information
RID/ADR
UN#: 1104
Class: 3
PG: III
Proper Shipping Name: Amyl acetates
IMDG
UN#: 1104
Class: 3
PG: III
Proper Shipping Name: Amyl acetates
Marine Pollutant: No
Severe Marine Pollutant: No
IATA
UN#: 1104
Class: 3
PG: III
Proper Shipping Name: Amyl acetates
Inhalation Packing Group I: No
15 - Regulatory Information
CLASSIFICATION AND LABELING ACCORDING TO EU DIRECTIVES
R-PHRASES: 10
Flammable.
S-PHRASES: 16
Keep away from sources of ignition - no smoking.
Caution: Substance not yet fully tested (EU).
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A