四乙烯基锡 | 1112-56-7
中文名称
四乙烯基锡
中文别名
——
英文名称
tetravinyltin (IV)
英文别名
tetravinyl tin;tetravinylstannane
CAS
1112-56-7
化学式
C8H12Sn
mdl
MFCD00008608
分子量
226.893
InChiKey
MZIYQMVHASXABC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:160-163 °C (lit.)
-
密度:1.246 g/mL at 25 °C (lit.)
-
闪点:105 °F
-
保留指数:914;914;914;911
-
稳定性/保质期:
- 避免与不相容材料接触。
- 与强氧化剂反应。
由于试剂分子中含有C-Sn键,因此具有剧毒性。建议在通风橱中小心操作和使用。
计算性质
-
辛醇/水分配系数(LogP):4.67
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
毒理性
神经毒素 - 其他中枢神经系统神经毒素
Neurotoxin - Other CNS neurotoxin
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
安全信息
-
TSCA:No
-
危险等级:6.1(a)
-
危险品标志:T
-
安全说明:S36/37/39,S45
-
危险类别码:R23/24/25,R10
-
WGK Germany:3
-
海关编码:2931900090
-
危险品运输编号:UN 1993 3/PG 3
-
包装等级:II
-
危险类别:6.1(a)
-
危险标志:GHS02,GHS06
-
危险性描述:H226,H301,H311,H331
-
危险性防范说明:P261,P280,P301 + P310,P311
-
储存条件:密封保存于阴凉、干燥的库房中,并远离火源和易燃易爆区域。
SDS
| Name: | Tetravinyl Tin Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 1112-56-7 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1112-56-7 | Tetravinyl Tin | 100 | 214-193-6 |
Risk Phrases: 10 23/24/25
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Toxic by inhalation, in contact with skin and if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Vapors may cause dizziness or suffocation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire.
Use water spray to keep fire-exposed containers cool. Containers may explode in the heat of a fire. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. For large fires, use water spray, fog, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Water may be ineffective. Do NOT use straight streams of water.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1112-56-7: United Kingdom, WEL - TWA: (listed as tin organic compounds): 0.1 mg/m3 TWA (except cyhexatin, as Sn) United Kingdom, WEL - STEL: (listed as tin organic compounds): 0.
mg/m3 STEL (except cyhexatin, as Sn) United States OSHA: 0.1 mg/m3 TWA (as Sn) (listed under Tin orga compounds).
Belgium - TWA: (listed as tin organic compounds): 0.1 mg/m3 VLE ( Sn) Belgium - STEL: (listed as tin organic compounds): 0.2 mg/m3 VLE Sn) France - VME: (listed as tin organic compounds): 0.1 mg/m3 VME (a Sn) France - VLE: (listed as tin organic compounds): 0.2 mg/m3 VLE (a Sn) Germany: (listed as tin organic compounds): 0.1 mg/m3 VME (as Sn) Germany: (listed as tin organic compounds): Skin absorber Malaysia: (listed as tin organic compounds): 0.1 mg/m3 TWA (as Sn Netherlands: (listed as tin organic compounds): 0.2 mg/m3 STEL (a Sn) Netherlands: (listed as tin organic compounds): 0.1 mg/m3 MAC (as Spain: (listed as tin organic compounds): 0.1 mg/m3 VLA-ED (as Sn Spain: (listed as tin organic compounds): 0.2 mg/m3 VLA-EC (as Sn Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear, colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 160 - 163 deg C
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 40 deg C ( 104.00 deg F)
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.2460g/cm3
Molecular Formula: Sn(CH=CH2)4
Molecular Weight: 226.88
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1112-56-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Tetravinyl Tin - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, TOXIC, N.O.S.*
Hazard Class: 3 (6.1)
UN Number: 1992
Packing Group: III
IMO
Shipping Name: FLAMMABLE LIQUID, TOXIC, N.O.S.
Hazard Class: 3.3 (6.1)
UN Number: 1992
Packing Group: III
RID/ADR
Shipping Name: FLAMMABLE LIQUID, TOXIC, N.O.S.
Hazard Class: 3 (6.1)
UN Number: 1992
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T
Risk Phrases:
R 10 Flammable.
R 23/24/25 Toxic by inhalation, in contact with skin
and if swallowed.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 28A After contact with skin, wash immediately with
plenty of water.
S 33 Take precautionary measures against static
discharges.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1112-56-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1112-56-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1112-56-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法
合成制备方法
同样地,四乙烯基锡也是通过乙烯基溴化镁与四氯化锡的反应来制备的。[1]
用途简介在有机合成中,四乙烯基锡(式1)主要用作含有乙烯官能团化合物和试剂的前体,并参与亲核反应,在底物中引入乙烯基。通常情况下,这种亲核反应是在有机锂试剂的帮助下进行的:四乙烯基锡首先与有机锂试剂发生交换生成乙烯基锂后再进一步反应。在相似条件下,四乙烯基锡还可以与醛酮反应生成亲核加成产物(式2)[2,3];与α,β-不饱和羰基化合物反应生成1,4-加成产物(式3)[4];与环氧反应生成开环产物(式4)[5~7]。另外,四乙烯基锡在金属钯催化剂存在下,能够与sp2碳原子上的卤原子或氟磺酸酯发生偶联反应[8~10]。多种类型的钯催化剂可用于该过程,在芳环上引入乙烯基(式5)。此外,四乙烯基锡还可以与具有活性氢的酚生成乙烯基醚产物,并且该反应需要氧气参与(式6)[11,12]。
用途在有机合成中,四乙烯基锡主要用作含有乙烯官能团化合物和试剂的前体,并通过亲核反应引入乙烯基。通常这种亲核反应是在有机锂试剂的帮助下进行:首先与有机锂试剂发生交换生成乙烯基锂后再进一步反应[2,3];四乙烯基锡还可与醛酮反应生成亲核加成产物(式2)[2,3],与α,β-不饱和羰基化合物生成1,4-加成产物(式3)[4],与环氧生成开环产物(式4)[5~7]。此外,在金属钯催化剂存在下,四乙烯基锡还能与sp2碳原子上的卤原子或氟磺酸酯发生偶联反应[8~10]。多种类型的钯催化剂可用于该过程,并在芳环上引入乙烯基(式5)。另外,四乙烯基锡还能够与具有活性氢的酚生成乙烯基醚产物,并且这一反应需要氧气参与(式6)[11,12]。
反应信息
-
作为反应物:描述:参考文献:名称:Organoboron Halides. II. The Vinylhaloboranes, A Preliminary Study of their Preparation and Properties1,2摘要:DOI:10.1021/ja01509a002
-
作为产物:参考文献:名称:The Preparation of Some Vinyltin Compounds with Vinylmagnesium Chloride摘要:DOI:10.1021/ja01566a028
-
作为试剂:描述:(5R,7R)-8-(benzyloxy)-N-methoxy-N,7-dimethyl-5-((triethylsilyl)oxy)octanamide 在 四乙烯基锡 、 甲基锂 作用下, 以 四氢呋喃 、 乙醚 为溶剂, 反应 1.5h, 以96%的产率得到(7R,9R)-9-methyl-10-phenylmethoxy-7-triethylsilyloxydec-1-en-3-one参考文献:名称:(-)-Exiguolide的全合成摘要:天然对映体(-)-exiguolide的全合成是首次完成。通过连续的烯烃交叉复分解/分子内的氧杂共轭物加成/还原性醚化有效地合成了双(四氢吡喃)亚基。通过山口宏观内酯化来实现20元大环的构建。通过Suzuki-Miyaura偶联立体选择性地引入(E,Z,E)-三烯侧链,从而完成了总合成过程。DOI:10.1021/ol902778y
文献信息
-
[EN] OXADIAZOLE COMPOUNDS WHICH INHIBIT BETA-SECRETASE ACTIVITY AND METHODS OF USE THEREOF<br/>[FR] COMPOSÉS OXADIAZOLE QUI INHIBENT L'ACTIVITÉ DE LA BÊTA-SECRÉTASE, ET LEURS PROCÉDÉS D'UTILISATION申请人:COMENTIS INC公开号:WO2012054510A1公开(公告)日:2012-04-26The invention provides novel beta-secretase inhibitors and methods for their including methods of treating Alzheimer's disease.这项发明提供了新型β-分泌酶抑制剂以及它们的方法,包括治疗阿尔茨海默病的方法。
-
Synthesis of 3-Aryl-1-aminopropane Derivatives: Lithiation–Borylation–Ring-Opening of Azetidinium Ions作者:Giorgia Casoni、Eddie Myers、Varinder AggarwalDOI:10.1055/s-0035-1562447日期:2016.10C–B bond of the γ-dimethylamino tertiary boronic esters can be transformed into a variety of functional groups (C–OH, C–vinyl, C–H, C–BF3), thus giving a diverse selection of 3-aryl-1-aminopropanes, which represent a privileged motif among drug molecules. In situ generated 2-phenyl-azetidinium ylides react with boronic esters to form acyclic γ-dimethylamino tertiary boronic esters. The transformation致力于纪念Jean Normant教授 抽象的 原位生成的2-苯基-氮杂环丁烷基鎓盐与硼酸酯反应形成无环的γ-二甲基氨基叔硼酸酯。据信该转化涉及两性离子硼酸酯的形成,其随后经历开环的1,2-迁移,其通过消除环应变而促进。由于最初形成的伊利德的构型不稳定性似乎与开链卡宾形式处于平衡状态,因此该反应不是立体特异性的。γ-二甲基氨基叔硼酸酯的C–B键可以转化为各种官能团(C–OH,C–乙烯基,CH–H,C–BF 3),从而提供了多种多样的3-芳基选择-1-氨基丙烷代表药物分子中的优先基序。 原位生成的2-苯基-氮杂环丁烷基鎓盐与硼酸酯反应形成无环的γ-二甲基氨基叔硼酸酯。据信该转化涉及两性离子硼酸酯的形成,其随后经历开环的1,2-迁移,其通过消除环应变而促进。由于最初形成的伊利德的构型不稳定性似乎与开链卡宾形式处于平衡状态,因此该反应不是立体特异性的。γ-二甲基氨基叔硼酸酯的C–B键可以转化为各
-
Synthesis applications of aza-Cope rearrangements. 12. Applications of cationic aza-Cope rearrangements for alkaloid synthesis. Stereoselective preparation of cis-3a-aryloctahydroindoles and a new short route to Amaryllidaceae alkaloids作者:Larry E. Overman、Leah T. Mendelson、E. Jon JacobsenDOI:10.1021/ja00360a014日期:1983.10Synthese d'aryl-3a perhydro indolinones-4(A) a partir d'amino-2 aryl-1' vinyl-1 cyclopentanols; application a la synthese de crinine par l'intermediaire de A avec aryl=benzodioxole-1,3yl-5合成这些 d'aryl-3a perhydroindolinones-4(A) a partir d'amino-2 aryl-1'vinyl-1 cyclopentanols; 应用 a la 合成 de crinine par l'intermediaire de A avec aryl=benzodioxole-1,3yl-5
-
Unsaturated σ-hydrocarbyl transition-metal complexes. Part 2. Synthesis and reactions of vinylplatinum complexes and a comparison with analogous fluorovinyl and alkynyl complexes作者:Christine J. Cardin、David J. Cardin、Michael F. LappertDOI:10.1039/dt9770000767日期:——cis-[PtRL2(SnMe3)]. When the latter complex (R = CHCH2) reacts with X2 or MeX further oxidative addition occurs exclusively at the platinum centre. Aromatic isonitriles (R′NC)co-ordinate to the platinum and give insertion products trans-[PtC(CHCH2)= NR′}ClL2] on heating or carbene complexes with NBunH2. The alkynyl trans-[Pt(CCPh)ClL2] also forms 1 :1 adducts with R′NC and carbene complexes therefrom, but no insertion三甲基锡的烯基(CH CH 2或CF CF 2)或炔基(C CPh)衍生物在通过复分解合成相应的单有机铂(II)物种方面优于锂或镁试剂(L = SnMe 3 R +顺式-[PtCl 2 L 2 ] →反式-[PtRClL 2 ] + SnMe 3 Cl叔膦)。SnMe 3 R的反应顺序为R = C CPh> CF CF 2 > CH CH 2。还发现该顺序用于氧化添加SnMe 3R至Pt 0给出顺式-[PtRL 2(SnMe 3)]。当后者的络合物(R = CH CH 2)与X 2或MeX反应时,进一步的氧化加成仅在铂中心发生。芳香异腈(R'NC)与铂配位,并在加热或带有NBu n H 2的卡宾配合物中得到插入产物反式-[Pt C(CH CH 2)= NR'} ClL 2 ] 。炔基反式-[Pt(C CPh)ClL 2]也与R'NC和卡宾配合物形成1:1加合物,但没有插入产物。给出了新配合物的光谱数据。
-
Discovery of Functionally Selective 7,8,9,10-Tetrahydro-7,10-ethano-1,2,4-triazolo[3,4-<i>a</i>]phthalazines as GABA<sub>A</sub> Receptor Agonists at the α<sub>3</sub> Subunit作者:Michael G. N. Russell、Robert W. Carling、John R. Atack、Frances A. Bromidge、Susan M. Cook、Peter Hunt、Catherine Isted、Matt Lucas、Ruth M. McKernan、Andrew Mitchinson、Kevin W. Moore、Robert Narquizian、Alison J. Macaulay、David Thomas、Sally-Anne Thompson、Keith A. Wafford、José L. CastroDOI:10.1021/jm040883v日期:2005.3.1We have previously identified the 7,8,9,10-tetrahydro-7,10-ethano-1,2,4-triazolo[3,4-a]phthalazine (1) as a potent partial agonist for the alpha(3) receptor subtype with 5-fold selectivity in binding affinity over alpha(1). This paper describes a detailed investigation of the substituents on this core structure at both the 3- and 6-positions. Despite evaluating a wide range of groups, the maximum selectivity我们之前已经确定了7,8,9,10-四氢-7,10-乙醇-1,2,4-三唑并[3,4-a]酞嗪(1)是α(3)的有效部分激动剂。受体亚型,对α(1)的结合亲和力具有5倍的选择性。本文描述了在此核心结构的3位和6位上的取代基的详细研究。尽管评估了广泛的组,但相对于alpha(1)亚型,对alpha(3)亚型的亲和力可达到的最大选择性是12倍(对于57)。尽管大多数类似物在功效上均未显示选择性,但一些类似物确实在α(1)处表现出部分激动作用,而在α(3)处表现出拮抗作用(例如25和75)。但是,测试了两个类似物(93和96),它们都在6位上有三唑取代基,显示出对alpha(3)亚型的疗效明显高于alpha(1)亚型。这是该系列中可以在所需方向上实现选择性的第一个迹象。
表征谱图
-
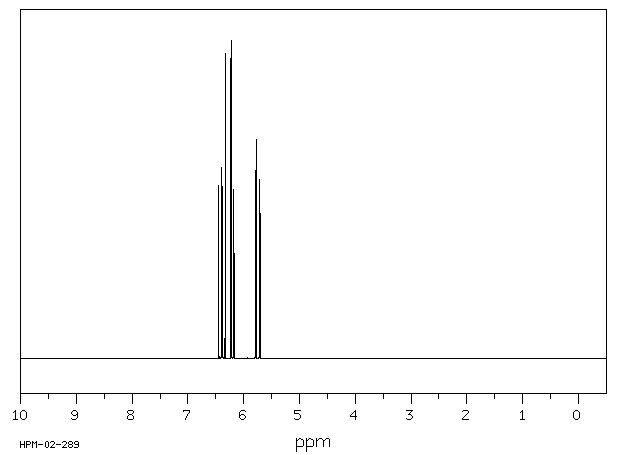
氢谱1HNMR
-
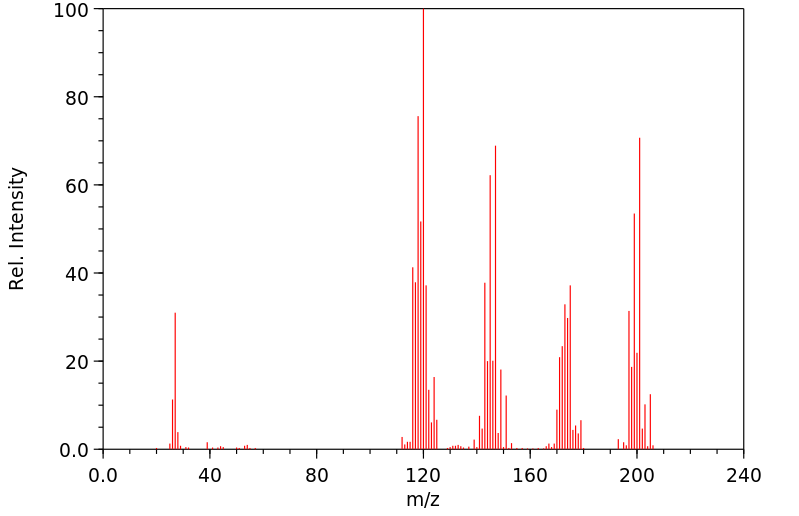
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锰,五羰基(三甲基甲锡烷基)-,(OC-6-22)-
锡烷,乙氧基三甲基-
锡烷,三丁基(1E)-1-庚烯基-
锡烷,三丁基(1-甲基-2-丁烯基)-,(Z)-
锡烷,三(1,1-二甲基乙基)乙炔基-
锡烷,(4-氯二环[2.2.1]庚-1-基)三甲基-
锡烷,(1E)-1-丁烯-3-炔基三丁基-
铝,三庚基-
铝,丁氧基二(2-甲基丙基)-
铅烷,三丁基-1-己炔基-
辛基锡
辛基氧代锡烷
膦,三(三甲基甲锡烷基)-
碳化铝
碘化三乙基铅
碘(三甲基)铅烷
硼烷胺,N,N-二(氯二甲基甲锡烷基)-1,1-二甲基-
硫烷负离子三甲基铅
硫代乙酸 S-[3-(三丁基锡烷基)丙基]酯
硒基二(三甲基锡)
癸酰(二羟基)铝
甲硫基三丁基锡烷
甲烷四基四(三甲基锡烷)
甲氧基二(2-甲基丙基)-铝
甲基锡
甲基烯丙基三正丁基锡
甲基氢化钼
甲基双(1-甲基环己基)锡烷
甲基二氯化铝
甲基三戊基锡
甲基(三丙基)锡烷
环己羧酸,2-氨基-,甲基酯,(1S,2S)-
环己基三异丙基锡烷
环己基[(三丁基锡烷基)氧基]重氮1-氧化物
环己基-三甲基锡烷
环己基(异丙基)二甲基锡烷
环丙基(三异丙基)锡烷
烯丙基三甲基锡烷
烯丙基三乙烯基锡烷
烯丙基三丁基锡
烯丙基三(3,3,4,4,5,5,6,6,7,7,8,8,8-十三氟辛基)锡烷
溴二乙基铝
溴三甲基铅
溴(异丙基)汞
溴(三乙基)铅
溴(三丁基)铅
氰酸三丁基锡烷
氯甲氧基甲基三丁基锡
氯甲基三甲基锡
氯化二己基铝