3-碘苯硼酸 | 221037-98-5
中文名称
3-碘苯硼酸
中文别名
3-碘代苯硼酸
英文名称
(3-iodophenyl)boronic acid
英文别名
3-iodobenzeneboronic acid;m-iodobenzene boronic acid;3-Iodophenylboronic acid
CAS
221037-98-5
化学式
C6H6BIO2
mdl
MFCD01319015
分子量
247.828
InChiKey
REEUXWXIMNEIIN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:195-197 °C (lit.)
-
沸点:358.8±44.0 °C(Predicted)
-
密度:1.95±0.1 g/cm3(Predicted)
-
稳定性/保质期:
遵循规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):2.63
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
安全说明:S26,S36,S37/39
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:2931900090
-
危险标志:GHS07
-
危险性描述:H302,H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:存放在0至6℃的阴凉干燥处。
SDS
1.1 产品标识符
: 3-Iodophenylboronic acid
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
危害类型象形图
信号词 警告
危险申明
H302 吞咽有害。
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/ 烟/ 气体/ 烟雾/ 蒸汽/ 喷雾。
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P302 + P352 如果在皮肤上: 用大量肥皂和水淋洗。
P304 + P340 如果吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如进入眼睛:用水小心清洗几分钟。如戴隐形眼镜并可方便地取出,取出
隐形眼镜。继续冲洗。
P312 如感觉不适,呼救解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P330 漱口。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/ 就诊。
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C6H6BIO2
分子式
: 247.83 g/mol
分子量
成分 浓度
3-Iodophenylboronic acid
-
化学文摘编号(CAS No.) 221037-98-5
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
在皮肤接触的情况下
用肥皂和大量的水冲洗。 请教医生。
在眼睛接触的情况下
用大量水彻底冲洗至少15分钟并请教医生。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 碘化氢, 硼烷/氧化硼
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据工业卫生和安全使用规则来操作。 休息以前和工作结束时洗手。
人身保护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 195 - 197 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 3-Iodophenylboronic acid
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
危害类型象形图
信号词 警告
危险申明
H302 吞咽有害。
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/ 烟/ 气体/ 烟雾/ 蒸汽/ 喷雾。
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P302 + P352 如果在皮肤上: 用大量肥皂和水淋洗。
P304 + P340 如果吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如进入眼睛:用水小心清洗几分钟。如戴隐形眼镜并可方便地取出,取出
隐形眼镜。继续冲洗。
P312 如感觉不适,呼救解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P330 漱口。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/ 就诊。
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C6H6BIO2
分子式
: 247.83 g/mol
分子量
成分 浓度
3-Iodophenylboronic acid
-
化学文摘编号(CAS No.) 221037-98-5
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
在皮肤接触的情况下
用肥皂和大量的水冲洗。 请教医生。
在眼睛接触的情况下
用大量水彻底冲洗至少15分钟并请教医生。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 碘化氢, 硼烷/氧化硼
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据工业卫生和安全使用规则来操作。 休息以前和工作结束时洗手。
人身保护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 195 - 197 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
反应信息
-
作为反应物:参考文献:名称:从有机三氟硼酸盐和缩醛合成二烷基醚摘要:在苛刻的碱性或酸性条件下通过 CO 键形成形成醚是有机化学中根深蒂固的合成断开。我们报告了一种战略替代方案,涉及 BF(3).OEt(2) 促进的稳定、易于制备的缩醛与广泛可用的芳基-、烯基-和炔基三氟硼酸钾的偶联。这种快速、操作简单的过程提供了直接获得二烷基醚的途径,其中许多使用经典方法难以制备。使用 MOM 保护的醇和缩醛保护的醛可以形成醚,而无需求助于保护基操作或强碱。DOI:10.1021/ja906514s
-
作为产物:参考文献:名称:新規化合物摘要:提供具有优异的CERT抑制活性且不具有与神经酰胺相似结构的新化合物。新化合物由结构式(I)组成。键基-X-为顺式环丙基,并且R1、R2、R3、R4和R5分别独立地为氢原子、卤素原子、可含有卤素原子的直链或支链的C1-C8烷基,或OR6。【选图】图1公开号:JP2019182779A
文献信息
-
一种铜催化的芳基硼酸与二氧化碳的羧化反 应方法
-
[EN] SMALL MOLECULE INHIBITORS OF NF-kB INDUCING KINASE<br/>[FR] INHIBITEURS À PETITE MOLÉCULE DE KINASE INDUISANT NF-KB申请人:JANSSEN PHARMACEUTICA NV公开号:WO2020239999A1公开(公告)日:2020-12-03The present invention relates to compounds that inhibit NIK and pharmaceutical compositions comprising such compounds and methods of using the same. These compounds and pharmaceutical compositions are envisaged to be useful for preventing or treating diseases such as cancer (such as B-cell malignancies including leukemias, lymphomas and myeloma), inflammatory disorders, autoimmune disorders, immunodermatologic disorders such as palmoplantar pustulosis and hidradenitis suppurativa, and metabolic disorders such as obesity and diabetes.本发明涉及抑制NIK的化合物,以及包含这些化合物的药物组合物和使用方法。这些化合物和药物组合物预期能用于预防或治疗癌症(如包括白血病、淋巴瘤和多发性骨髓瘤的B细胞恶性肿瘤)、炎症性疾病、自身免疫疾病、免疫皮肤病学疾病(如掌跖脓疱病和化脓性汗腺炎)以及代谢紊乱疾病(如肥胖和糖尿病)。
-
Synthesis of an Azabicyclo[3.1.0]hexanone-Containing Inhibitor of NF-κΒ Inducing Kinase via Catalytic C–H Activation作者:James J. Crawford、Daohong Liao、Aleksandr Kolesnikov、Wendy Lee、Matthew L. LandryDOI:10.1055/s-0040-1707279日期:2020.11
Abstract The synthesis of an azabicyclo[3.1.0]hexanone-containing inhibitor of the nuclear factor-κB inducing kinase (NIK) is reported. The initial route to this compound was streamlined from 13 to 7 linear steps through the use of a catalytic, enantioselective C–H activation step. A procedure for lactam oxidation was identified that avoided use of peroxides on scale. These synthetic improvements allowed for the synthesis of multigram quantities of the desired NIK inhibitor for in vivo profiling.
-
Structure–Activity Relationship for Thiirane-Based Gelatinase Inhibitors作者:Mijoon Lee、Masahiro Ikejiri、Dennis Klimpel、Marta Toth、Mana Espahbodi、Dusan Hesek、Christopher Forbes、Malika Kumarasiri、Bruce C. Noll、Mayland Chang、Shahriar MobasheryDOI:10.1021/ml300050b日期:2012.6.14inhibition. However, para- and some meta-substitutions of the terminal phenyl ring enhanced inhibitory activity and led to improve metabolic stability. This agrees with the result from metabolism studies with compound 1 that the primary route of biotransformation is oxidation, mainly at the para position of the phenyl ring and the α position of the sulfonyl group in the aliphatic side chain.
-
Comparison of Copper(II) Acetate Promoted N-Arylation of 5,5-Dimethyl Hydantoin and Other Imides with Triarylbismuthanes and Aryl Boronic Acids
表征谱图
-
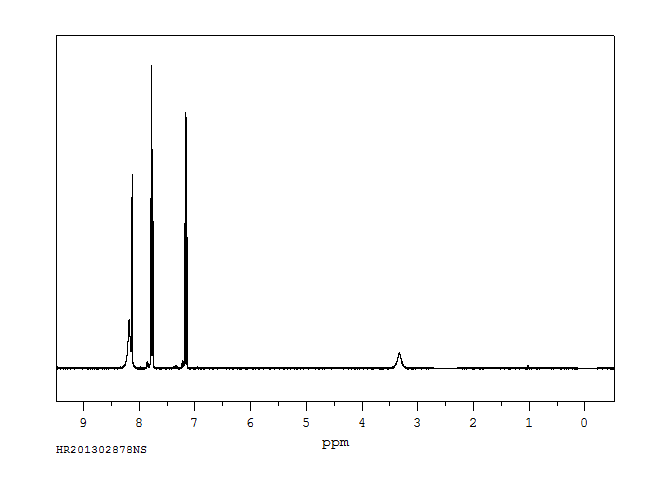
氢谱1HNMR
-
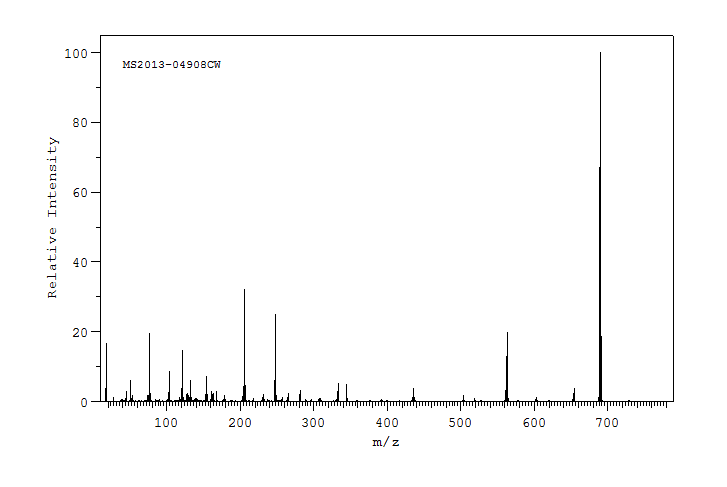
质谱MS
-
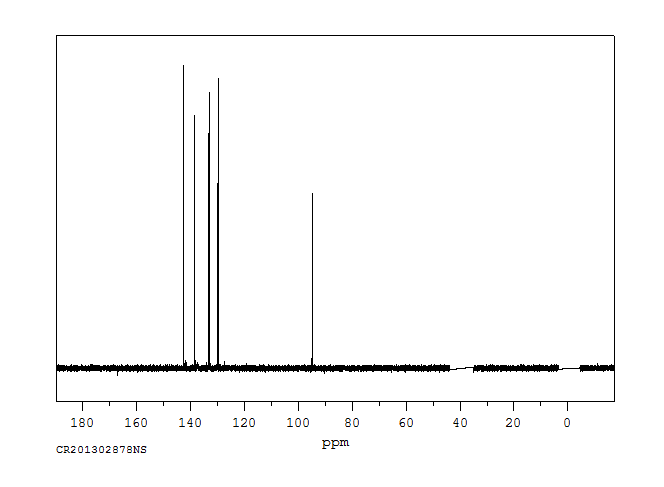
碳谱13CNMR
-
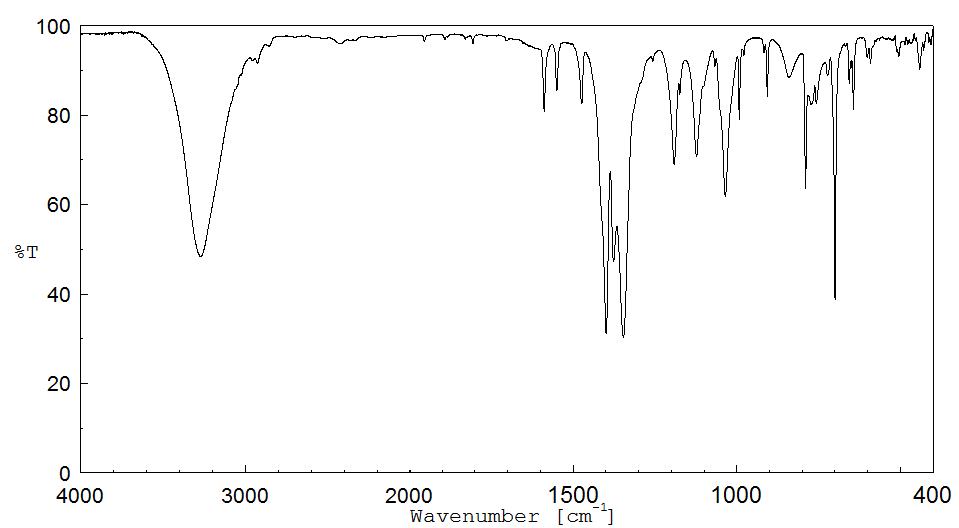
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫