1,3-苯并二唑-5-基异硫氰酸酯 | 113504-93-1
分子结构分类
中文名称
1,3-苯并二唑-5-基异硫氰酸酯
中文别名
5-异硫代氰酰基-1,3-苯并二氧代
英文名称
5-isothiocyanato-benzo[1,3]dioxole
英文别名
3,4-methylenedioxyphenyl isothiocyanate;5-isothiocyanatobenzo[d][1,3]dioxole;3,4-methylenedioxophenyl isothiocyanate;5-isothiocyanato-1,3-benzodioxole
CAS
113504-93-1
化学式
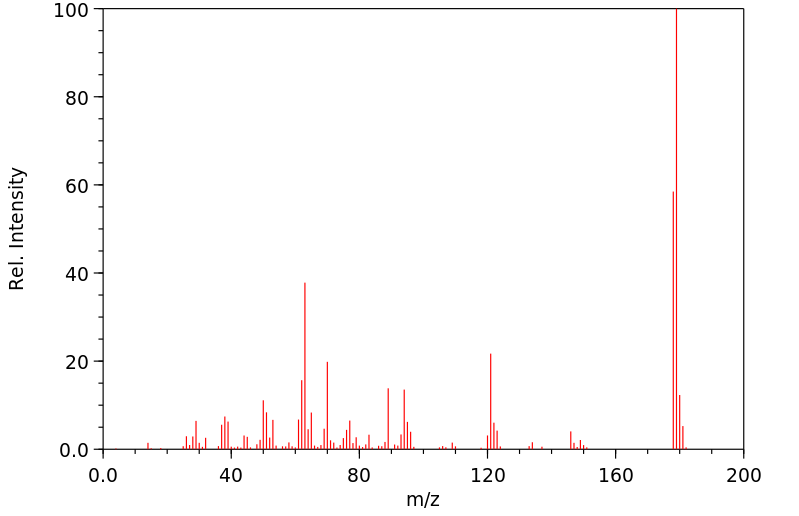
C8H5NO2S
mdl
MFCD00066321
分子量
179.199
InChiKey
UVVSPZKAEJHDCY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:57 °C
-
沸点:137 °C
-
密度:1.37±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:62.9
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi,T
-
危险类别码:R20/21/22
-
危险品运输编号:UN 2811
-
海关编码:2932999099
-
危险类别:IRRITANT
-
安全说明:S26,S36/37/39
-
储存条件:保持冷静
SDS
| Name: | 1 3-Benzodioxol-5-yl isothiocyanate 97% Material Safety Data Sheet |
| Synonym: | 3,4-Methylenedioxyphenyl isothiocynaat |
| CAS: | 113504-93-1 |
Synonym:3,4-Methylenedioxyphenyl isothiocynaat
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 113504-93-1 | 1,3-Benzodioxol-5-yl isothiocyanate | 97% | unlisted |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.Moisture sensitive.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Store under an inert atmosphere.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 113504-93-1: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: tan
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H5NO2S
Molecular Weight: 179
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials, exposure to moist air or water.
Incompatibilities with Other Materials:
Amines, strong oxidizing agents, alcohols.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, oxides of sulfur, carbon dioxide, acrid smoke and fumes.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 113504-93-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,3-Benzodioxol-5-yl isothiocyanate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 113504-93-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 113504-93-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 113504-93-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4-亚甲二氧基苯胺 benzo[1,3]dioxolo-5-ylamine 14268-66-7 C7H7NO2 137.138 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(3,4-亚甲基二氧苯基)-2-硫脲 1-(benzo[d][1,3]dioxol-5-yl)thiourea 65069-55-8 C8H8N2O2S 196.23 4-(3,4-亚甲基二氧基苯基)-3-氨基硫脲 4-(1,3-benzodioxol-5-yl)thiosemicarbazide 206761-71-9 C8H9N3O2S 211.244
反应信息
-
作为反应物:描述:参考文献:名称:Design, Synthesis, X-ray Crystallographic Analysis, and Biological Evaluation of Thiazole Derivatives as Potent and Selective Inhibitors of Human Dihydroorotate Dehydrogenase摘要:Human dihydroorotate dehydrogenase (HsDHODH) is a flavin-dependent mitochondrial enzyme that has been certified as a potential therapeutic target for the treatment of rheumatoid arthritis and other autoimmune diseases. On the basis of lead compound 4, which was previously identified as potential HsDHODH inhibitor, a novel series of thiazole derivatives were designed and synthesized. The X-ray complex structures of the promising analogues 12 and 33 confirmed that these inhibitors bind at the putative ubiquinone binding tunnel and guided us to explore more potent inhibitors, such as compounds 44, 46, and 47 which showed double digit nanomolar activities of 26, 18, and 29 nM, respectively. Moreover, 44 presented considerable anti-inflammation effect in vivo and significantly alleviated foot swelling in a dose-dependent manner, which disclosed that thiazole-scaffold analogues can be developed into the drug candidates for the treatment of rheumatoid arthritis by suppressing the bioactivity of HsDHODH.DOI:10.1021/jm501127s
-
作为产物:描述:参考文献:名称:2-氨基恶唑的微波介导合成摘要:开发了在 150 °C 下微波介导的 2-氨基恶唑合成,提供了具有多种官能团的产品。反应需要 5 分钟,并以中等至良好的收率提供具有简单沉淀的产物,无需重结晶或快速色谱法。DOI:10.1016/j.tetlet.2021.153555
文献信息
-
Discovery of SHR1653, a Highly Potent and Selective OTR Antagonist with Improved Blood–Brain Barrier Penetration作者:Xin Li、Zhigao Zhang、Yang Chen、Hong Wan、Jiakang Sun、Bin Wang、Bingqiang Feng、Bing Hu、Xingxing Shi、Jun Feng、Lei Zhang、Feng He、Chang Bai、Lianshan Zhang、Weikang TaoDOI:10.1021/acsmedchemlett.9b00186日期:2019.6.13oxytocin receptor (OTR) plays a major role in the control of male sexual responses. Antagonists of the OTR have been reported to inhibit ejaculation in animal models and serve as a potential treatment for premature ejaculation (PE). Herein, we describe a novel scaffold featuring an aryl substituted 3-azabicyclo [3.1.0] hexane structure. The lead compound, SHR1653, was shown to be a highly potent OTR antagonist催产素受体(OTR)在控制男性性反应中起主要作用。据报道,在动物模型中,OTR的拮抗剂可抑制射精,并可作为早泄(PE)的潜在治疗方法。在这里,我们描述了一种新型的脚手架,其特征是芳基取代的3-氮杂双环[3.1.0]己烷结构。铅化合物SHR1653被证明是一种高效的OTR拮抗剂,相对于V 1A R,V 1B R和V 2 R表现出优异的选择性。该新型分子也显示出对整个物种都具有良好的药代动力学特征体内一样健壮在大鼠子宫收缩模型中的功效。有趣的是,SHR1653表现出出色的血脑屏障穿透性,可能对中枢神经系统相关PE的治疗有益。
-
An automated, polymer-assisted strategy for the preparation of urea and thiourea derivatives of 15-membered azalides as potential antimalarial chemotherapeutics作者:Antun Hutinec、Renata Rupčić、Dinko Žiher、Kirsten S. Smith、Wilbur Milhous、William Ellis、Colin Ohrt、Zrinka Ivezić SchönfeldDOI:10.1016/j.bmc.2011.01.030日期:2011.3series of 15-membered azalide urea and thiourea derivatives has been synthesized and evaluated for their in vitro antimalarial activity against chloroquine-sensitive (D6), chloroquine/pyremethamine resistant (W2) and multidrug resistant (TM91C235) strains of Plasmodium falciparum. We have developed an effective automated synthetic strategy for the rapid synthesis of urea/thiourea libraries of a macrolide
-
Synthesis and evaluation of new thiourea derivatives as antitumor and antiangiogenic agents作者:Wenjing Bai、Jianxin Ji、Qiang Huang、Wei WeiDOI:10.1016/j.tetlet.2020.152366日期:2020.10A series of novel thiourea derivatives were synthesized and evaluated by biological activities. Among them, compound 10e containing 3,5-bis(trifluoromethyl)phenyl moiety (R1) at the terminal thiourea and phenylamino (R2) at the terminal acyl position showed the best cytotoxic activities against seven cancer cell lines (NCI-H460, Colo-205, HCT116, MDA-MB-231, MCF-7, HepG2, PLC/PRF/5) and HUVECs. Moreover
-
[EN] CYANOGUANIDINES AND THEIR USE AS ANTIVIRAL AGENTS<br/>[FR] CYANOGUANIDINES ET LEUR UTILISATION COMME AGENTS ANTIVIRAUX申请人:ABBVIE INC公开号:WO2014005129A1公开(公告)日:2014-01-03This disclosure relates to: (a) compounds and salts thereof that, inter alia, inhibit RSV infection and/or replication; (b) intermediates useful for the preparation of such compounds and salts; (c) compositions comprising such compounds and salts; (d) methods for preparing such intermediates, compounds, salts, and compositions; (e) methods of use of such compounds, salts, and compositions; and (f) kits comprising such compounds, salts, and compositions.这份披露涉及:(a) 抑制RSV感染和/或复制的化合物及其盐,等等;(b) 用于制备这些化合物和盐的中间体;(c) 包含这些化合物和盐的组合物;(d) 制备这些中间体、化合物、盐和组合物的方法;(e) 使用这些化合物、盐和组合物的方法;以及(f) 包含这些化合物、盐和组合物的试剂盒。
-
Design, Synthesis, and Structure−Activity Relationship of Substrate Competitive, Selective, and in Vivo Active Triazole and Thiadiazole Inhibitors of the c-Jun N-Terminal Kinase作者:Surya K. De、John L. Stebbins、Li-Hsing Chen、Megan Riel-Mehan、Thomas Machleidt、Russell Dahl、Hongbin Yuan、Aras Emdadi、Elisa Barile、Vida Chen、Ria Murphy、Maurizio PellecchiaDOI:10.1021/jm801503n日期:2009.4.9We report comprehensive structure−activity relationship studies on a novel series of c-Jun N-terminal kinase (JNK) inhibitors. The compounds are substrate competitive inhibitors that bind to the docking site of the kinase. The reported medicinal chemistry and structure-based optimizations studies resulted in the discovery of selective and potent thiadiazole JNK inhibitors that display promising in
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
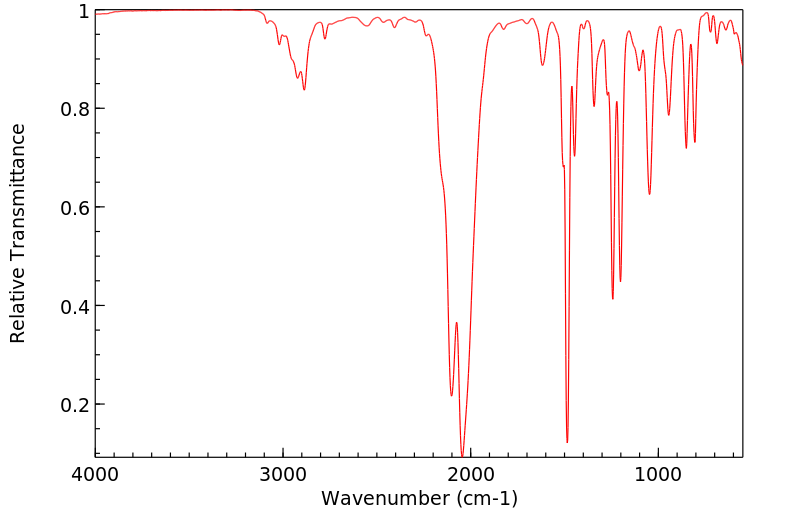
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5-(4-乙氧基-3-甲基苄基)-1,3-苯并二恶茂)
黄樟素氧化物
黄樟素乙二醇; 2',3'-二氢-2',3'-二羟基黄樟素
黄樟素
风藤酰胺
风藤酮
非哌西特盐酸盐
非哌西特 盐酸盐
角秋水仙碱
螺[1,3-苯并二氧戊环-2,1'-环己烷]-5-胺
蓝细菌
苯并[d][1,3]二氧杂环戊烯-5-胺盐酸盐
苯并[d][1,3]二氧代l-5-甲基(2-氧代乙基)氨基甲酸叔丁酯
苯并[d][1,3]二氧代l-5-氨基甲酸叔丁酯
苯并[d][1,3]二氧代-4-甲腈
苯并[d][1,3]二氧代-4-氨基甲酸叔丁酯
苯并[d[1,3]二氧代-4-羧酰胺
苯并[1,3]二氧杂环戊烯-5-基甲基2-氯乙酸酯
苯并[1,3]二氧杂环戊烯-5-基甲基-苄基-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-[2-(4-氟-苯基)-乙基]-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-(四氢-呋喃-2-基甲基)-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-(2-氟-苄基)-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-(1-甲基-哌啶-4-基)-胺
苯并[1,3]二氧代l-5-甲基-吡啶-3-甲基-胺
苯并[1,3]二氧代l-5-甲基-(4-氟-苄基)-胺
苯并[1,3]二氧代l-5-乙酸甲酯
苯并[1,3]二氧代-5-羧酰胺盐酸盐
苯并[1,3]二氧代-5-甲基肼盐酸盐
苯并[1,3]二氧代-5-甲基吡啶-4-甲胺
苯并[1,3]二氧代-5-甲基-吡啶-2-甲胺
苯并[1,3]二氧代-5-乙酰氯
苯并-1,3-二氧杂环戊烯-5-甲醇丙酸酯
苯乙酸,1-(1,3-苯并二氧杂环戊烯-5-基)-3-丁烯-1-基酯
苯乙酮O-((4-(3,4-亚甲二氧基苄基)-1-哌嗪-1-基)羰基甲基)肟
苯,1-甲氧基-6-硝基-3,4-亚甲二氧基-
芝麻酚
芝麻林素
脲,N-1,3-苯并二噁唑-5-基-N'-(2-溴乙基)-
胡椒醛肟
胡椒醛-((Z)-O-苯基氨基甲酰基肟)
胡椒醛,二苄基缩硫醛
胡椒醛
胡椒醇
胡椒酸酰氯
胡椒酸
胡椒腈
胡椒环乙酮肟
胡椒环
胡椒基重氮酮
胡椒基甲醛