1-苯并噻吩-5-甲醛 | 10133-30-9
中文名称
1-苯并噻吩-5-甲醛
中文别名
苯并[B]噻吩-5-甲醛
英文名称
benzo[b]thiophene-5-carbaldehyde
英文别名
1-benzothiophene-5-carbaldehyde;benzo[b]thiophene-5-carboxaldehyde
CAS
10133-30-9
化学式
C9H6OS
mdl
MFCD05663673
分子量
162.212
InChiKey
QHHRWAPVYHRAJA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:53 °C
-
沸点:303.2±15.0 °C(Predicted)
-
密度:1.300±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:11
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:45.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P304+P340,P305+P351+P338
-
危险性描述:H302,H312,H315,H319,H332,H335
-
储存条件:存储条件:2-8℃,需使用惰性气体保护。
SDS
| Name: | 1-Benzothiophene-5-carbaldehyde Material Safety Data Sheet |
| Synonym: | None know |
| CAS: | 10133-30-9 |
Synonym:None know
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 10133-30-9 | 1-Benzothiophene-5-carbaldehyde | 95+% | unlisted |
Risk Phrases: 22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful if swallowed. Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Causes respiratory tract irritation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid generating dusty conditions.
Section 7 - HANDLING and STORAGE
Handling:
Use with adequate ventilation. Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 10133-30-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 56 - 57 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H6OS
Molecular Weight: 162.21
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, reducing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 10133-30-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-Benzothiophene-5-carbaldehyde - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 22 Harmful if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 22 Do not breathe dust.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 10133-30-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 10133-30-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 10133-30-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-甲基苯并噻吩 5-methylthianaphthene 14315-14-1 C9H8S 148.229 1-苯并噻吩-5-羧酸 benzo[b]thiophene-5-carboxylic acid 2060-64-2 C9H6O2S 178.211 1-苯并噻吩-5-甲醇 5-(hydroxymethyl)benzothiophene 20532-34-7 C9H8OS 164.228 —— 5-bromomethyl-benzo[b]thiophene 10133-22-9 C9H7BrS 227.125 1-苯并噻吩-5-腈 benzo[b]thiophene-5-carbonitrile 2060-63-1 C9H5NS 159.211 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-bromomethyl-benzo[b]thiophene 10133-22-9 C9H7BrS 227.125 —— 3-bromobenzo[b]thiophene-5-carbaldehyde 10135-01-0 C9H5BrOS 241.108 —— 4-benzo[β]thiophen-5-ylbut-3-en-2-one 95353-38-1 C12H10OS 202.277 —— trans-4-(benzo[b]thiophen-5-yl)-but-3-en-2-one 329768-76-5 C12H10OS 202.277 —— (E)-ethyl 3-(benzo[b]thiophen-5-yl)acrylate 921219-04-7 C13H12O2S 232.303 2-(苯并[b]噻吩-5-基)-1,3-二氧戊环 5-(1,3-dioxolan-2-yl)benzo[b]thiophene 96803-06-4 C11H10O2S 206.265 —— 5-(1,2-epoxyethyl)benzothiophene 131965-81-6 C10H8OS 176.239 —— α-(benzo[b]thiophen-5-yl)-1-benzeneethanamine 170688-97-8 C16H15NS 253.368
反应信息
-
作为反应物:描述:参考文献:名称:[EN] SPIRO-OXADIAZOLINE COMPOUNDS AS AGONISTS OF α-7-NICOTINIC ACETYLCHOLINE RECEPTORS
[FR] COMPOSÉS SPIRO-OXADIAZOLINE EN TANT QU'AGONISTES DES RÉCEPTEURS DE L'ACÉTYLCHOLINE Α-7 NICOTINIQUE摘要:本发明涉及新型螺环-噁二唑啉化合物,适用作a7-nAChR的激动剂或部分激动剂,以及这些化合物和组合物的制备方法、药物组合物,以及在维持、治疗和/或改善认知功能的方法中使用这些化合物和组合物。具体而言,涉及向需要的患者(例如患有认知缺陷和/或希望增强认知功能的患者)施用螺环-噁二唑啉cx7-nAChR激动剂或部分激动剂的方法,以使其获益。公开号:WO2015066371A1 -
作为产物:描述:5-溴苯并[b]噻吩 在 manganese(IV) oxide 、 lithium aluminium tetrahydride 、 四(三苯基膦)钯 、 水 、 lithium hydroxide 作用下, 以 四氢呋喃 、 二氯甲烷 、 N,N-二甲基甲酰胺 为溶剂, 反应 3.0h, 生成 1-苯并噻吩-5-甲醛参考文献:名称:GAMMA-DIKETONES AS WNT/BETA -CATENIN SIGNALING PATHWAY ACTIVATORS摘要:本公开提供了激活Wnt/β-连环蛋白信号通路并因此治疗或预防与信号转导相关的疾病的γ-二酮或其类似物;这些疾病包括骨质疏松症和骨关节病;骨发育不全、骨缺陷、骨折、牙周病、耳硬化症、伤口愈合、颅颌面缺陷、溶骨性骨病、创伤性脑损伤或脊柱损伤、与中枢神经系统分化和发育相关的脑萎缩/神经系统疾病,包括帕金森病、中风、缺血性脑疾病、癫痫、阿尔茨海默病、抑郁症、躁郁症、精神分裂症;耳部疾病如耳蜗毛细胞丧失;眼部疾病如老年性黄斑变性、糖尿病性黄斑水肿或视网膜色素变性以及与干细胞分化和生长相关的疾病,如脱发、造血相关疾病和组织再生相关疾病。公开号:US20140243349A1
文献信息
-
Studies on Cognitive Enhancing Agents. III. Antiamnestic and Antihypoxic Activities of a Series of l-Bicycloaryl-2-(.OMEGA.-aminoalkoxy)ethanols.作者:Satoshi ONO、Tetsuo YAMAFUJI、Hisaaki CHAKI、Hajime MORITA、Yozo TODO、Naomi OKADA、Mutsuko MAEKAWA、Kazunori KITAMURA、Masaru TAI、Hirokazu NARITADOI:10.1248/cpb.43.1492日期:——2-(2-Aminoethoxy)-1-hydroxyethyl derivatives of bicyclic arenes) naphthalene, thianaphthene, benzofuran, and indole) were prepared and screened for antiamnestic (AA) and antihypoxic (AH) activities which were evaluated by measuring the reversing potency in electroconvulsion-induced amnesia and the protective effect against hypoxia, respectively, in mice. Compound 3o, 1-(benzo[b]thiophen-5-yl)-2-(2-diethylaminoethoxy)ethanol, showed the best AA and AH activity profile, being superior to our prototype compound, 2-(2-dimethylaminoethoxy)-1-phenylethanol (1). Elongation of the ethylene linkage in the side chain of 3o to 3- and 4-carbon moiethies brought about a significant decrease in AH activity. Compound 3o was further investigated for its protective effect against Co2-induced memory impairment and for acute toxicity in mice. It is ten-fold more potent than tacrine in the amnesia-reversal assay and is considerably less toxic than tacrine.
-
Generation of Phosphoranyl Radicals via Photoredox Catalysis Enables Voltage–Independent Activation of Strong C–O Bonds作者:Erin E. Stache、Alyssa B. Ertel、Tomislav Rovis、Abigail G. DoyleDOI:10.1021/acscatal.8b03592日期:2018.12.7oxygen-centered nucleophile. We show the desired reactivity in the reduction of benzylic alcohols to the corresponding benzyl radicals with terminal H atom trapping to afford the deoxygenated products. Using the same method, we demonstrate access to synthetically versatile acyl radicals, which enables the reduction of aromatic and aliphatic carboxylic acids to the corresponding aldehydes with exceptional chemoselectivity尽管醇和羧酸作为有机分子中的官能团盛行,并且有可能用作自由基前体,但C-O键仍然难以激活。我们报告了通过光氧化还原催化直接从这些普遍存在的官能团同时进入烷基和酰基自由基的合成策略。该方法利用了磷化氢自由基的独特反应性,该反应是由膦自由基阳离子和以氧为中心的亲核试剂之间的极性/ SET交叉产生的。我们显示了在末端醇被俘获以提供脱氧产物的情况下,将苄醇还原为相应的苄基所需要的反应性。使用相同的方法,我们演示了合成通用的酰基自由基的获得方法,可以将芳香族和脂肪族羧酸还原为相应的醛,并具有出色的化学选择性。该协议还通过分子内酰基自由基环化将羧酸转化为杂环和环状酮,从而一步一步形成C–O,C–N和C–C键。
-
Inhibition of the Cysteine Protease Human Cathepsin L by Triazine Nitriles: Amide⋅⋅⋅Heteroarene π-Stacking Interactions and Chalcogen Bonding in the S3 Pocket作者:Maude Giroud、Jakov Ivkovic、Mara Martignoni、Marianne Fleuti、Nils Trapp、Wolfgang Haap、Andreas Kuglstatter、Jörg Benz、Bernd Kuhn、Tanja Schirmeister、François DiederichDOI:10.1002/cmdc.201600563日期:2017.2.3We report an extensive “heteroarene scan” of triazine nitrile ligands of the cysteine protease human cathepsin L (hCatL) to investigate π‐stacking on the peptide amide bond Gly67–Gly68 at the entrance of the S3 pocket. This heteroarene⋅⋅⋅peptide bond stacking was supported by a co‐crystal structure of an imidazopyridine ligand with hCatL. Inhibitory constants (Ki) are strongly influenced by the diverse我们报道了半胱氨酸蛋白酶人组织蛋白酶L(hCatL)的三嗪腈配体的广泛的“杂芳烃扫描”,以研究S3口袋入口处的肽酰胺键Gly67–Gly68上的π堆积。杂芳基·····肽键的堆叠由咪唑并吡啶配体与hCatL的共晶体结构支持。抑制常数(ķ我)受到杂环的多样性和与S3口袋局部环境的特定相互作用的强烈影响。结合亲和力变化三个数量级。与烃类似物相比,所有杂芳族配体均具有增强的结合力。从杂芳烃和肽键的局部偶极矩的方向预测的能量贡献无法得到证实。分子间的C-S⋅⋅⋅O= C相互作用(硫族元素键)与Asn66的主链C = O增强了苯并噻吩基(K i = 4 n m)和苯并噻唑基(K i = 17 n m)配体的结合。 S3口袋。还测试了配体的相关酶罗德沙星。
-
Access to Spirocyclic Benzothiophenones with Multiple Stereocenters via an Organocatalytic Cascade Reaction作者:Bedřich Formánek、Jiří Tauchman、Ivana Císařová、Jan VeselýDOI:10.1021/acs.joc.0c00882日期:2020.7.2derivatives containing three stereocenters were prepared via one-step synthesis in yields ranging from 88 to 96% and in enantioselectivities (enantiomeric excess (ee)) ranging from 85 to 97%, with diastereoselectivities of approximately 14/2/1. Therefore, this method provides an efficient route for the synthesis of a new class of optically active 2-spirobenzothiophenones.
-
Asymmetric Catalysis on the Nanoscale: The Organocatalytic Approach to Helicenes作者:Lisa Kötzner、Matthew J. Webber、Alberto Martínez、Claudia De Fusco、Benjamin ListDOI:10.1002/anie.201400474日期:2014.5.12The first asymmetric organocatalytic synthesis of helicenes is reported. A novel SPINOL‐derived phosphoric acid, bearing extended π‐substituents, catalyzes the asymmetric synthesis of helicenes through an enantioselective Fischer indole reaction. A variety of azahelicenes and diazahelicenes could be obtained with good to excellent yields and enantioselectivities.
表征谱图
-
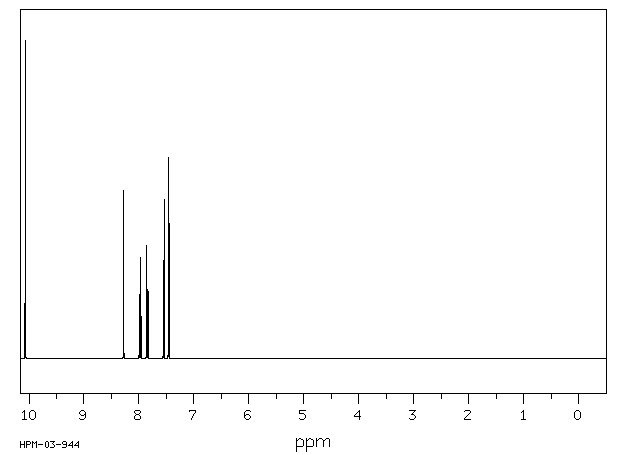
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
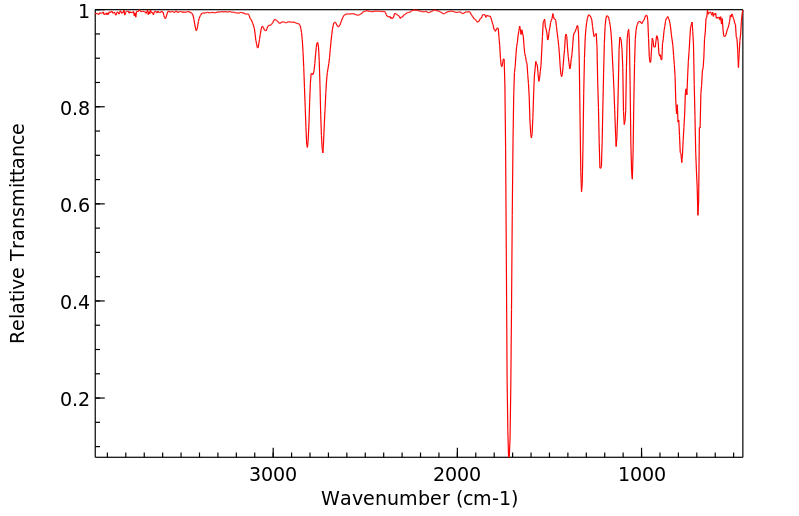
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐留通钠
齐留通相关物质A
齐留通亚砜
齐留通-d4
齐留通
雷洛昔芬杂质
邻联甲苯胺砜
试剂4,8-Bis(3,5-dioctyl-2-thienyl)-2,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[1,2-b:4,5-b']dithiophene
试剂1,1'-[4,8-Bis[4-(2-ethylhexyl)-3,5-difluorophenyl]benzo[1,2-b:4,5-b']dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]
苯并噻吩-7-醇
苯并噻吩-4-硼酸频哪醇酯
苯并噻吩-3-羧酸甲酯
苯并噻吩-3-硼酸
苯并噻吩-2-羰酰氯
苯并噻吩-2-羧酸肼
苯并噻吩-2-羧酸
苯并噻吩-2-硼酸
苯并噻吩-2-氨基甲酸叔丁酯
苯并噻吩
苯并[c]噻吩
苯并[b]噻吩-7-胺
苯并[b]噻吩-7-羧酸乙酯
苯并[b]噻吩-7-甲醛
苯并[b]噻吩-7-甲腈
苯并[b]噻吩-6-醇
苯并[b]噻吩-6-胺
苯并[b]噻吩-6-羧酸乙酯
苯并[b]噻吩-6-羧酸
苯并[b]噻吩-6-甲腈
苯并[b]噻吩-5-甲腈,2-甲酰基-
苯并[b]噻吩-5-甲磺酰氯
苯并[b]噻吩-4-羧酸甲酯
苯并[b]噻吩-4-羧酸
苯并[b]噻吩-4-甲醛
苯并[b]噻吩-4-甲腈
苯并[b]噻吩-4-基甲醇
苯并[b]噻吩-3-胺盐酸盐
苯并[b]噻吩-3-胺
苯并[b]噻吩-3-羧酸-(2-二烯丙基氨基乙酯)
苯并[b]噻吩-3-硼酸频哪酯
苯并[b]噻吩-3-甲醛肟
苯并[b]噻吩-3-甲酰胺
苯并[b]噻吩-3-基乙酸酯
苯并[b]噻吩-3-乙酸
苯并[b]噻吩-3-乙酰氯
苯并[b]噻吩-3-乙腈
苯并[b]噻吩-2-胺盐酸盐
苯并[b]噻吩-2-羧酸6-氨基-3-氯-甲酯
苯并[b]噻吩-2-羧酸,5-氯-3-(1-甲基乙氧基)-
苯并[b]噻吩-2-羧酸,3-羟基-5-甲氧基-,甲基酯