双乙酮 | 77-04-3
中文名称
双乙酮
中文别名
吡啶乙二酮;吡乙二酮
英文名称
3,3-diethyl-2,4(1H,3H)-pyridinedione
英文别名
Pyrithyldione;Didropyridin;Benedorm;3,3-diethyl-1H-pyridine-2,4-dione
CAS
77-04-3
化学式
C9H13NO2
mdl
MFCD00006010
分子量
167.208
InChiKey
NZASCBIBXNPDMH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:92-93°; mp 97-98°; mp 81-86° [Scheibl, Wachter, Arch. Toxikol. 18, 253 (1960)]
-
沸点:187-189 °C (14 mmHg)
-
密度:1.1222 (rough estimate)
-
溶解度:二氯甲烷;乙酸乙酯;甲醇
-
保留指数:1520;1510;1530;1557
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.555
-
拓扑面积:46.2
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
危险类别码:R20/21
-
安全说明:S23
SDS
| Name: | Pyrithyldione 98% Material Safety Data Sheet |
| Synonym: | Didropyridine; 3,3-Diethyl-2,4-pyridinedione; 3,3-Diethyl-2,4-(1H,3H)-pyridinedione; Dihydroprylone; Pyridione; Pyrithyldion |
| CAS: | 77-04-3 |
Synonym:Didropyridine; 3,3-Diethyl-2,4-pyridinedione; 3,3-Diethyl-2,4-(1H,3H)-pyridinedione; Dihydroprylone; Pyridione; Pyrithyldion
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 77-04-3 | Pyrithyldione | 98 | 201-000-5 |
Risk Phrases: 20/21
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation and in contact with skin.Harmful.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed. May cause central nervous system depression.
Inhalation:
The toxicological properties of this substance have not been fully investigated. May cause irritation of the mucous membranes.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of soap and water.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Keep containers tightly closed. Store in a cool, dry area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 77-04-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: none reported
pH: Acidic in aq soln.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 187.0 - 189.0 deg C @ 14.00mm
Freezing/Melting Point: 81-86 deg C, 92-93 deg C,
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Moderately Soluble.
Specific Gravity/Density: Not available.
Molecular Formula: C9H13NO2
Molecular Weight: 167.21
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 77-04-3: UT2810530 LD50/LC50:
CAS# 77-04-3: Oral, rat: LD50 = 780 mg/kg.
Carcinogenicity:
Pyrithyldione - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21 Harmful by inhalation and in contact with
skin.
Safety Phrases:
S 23 Do not inhale gas/fumes/vapour/spray.
WGK (Water Danger/Protection)
CAS# 77-04-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 77-04-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 77-04-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,3-二乙基-1-甲基-2,4(1H,3H)-吡啶二酮 3,3-diethyl-1-methyl-1H-pyridine-2,4-dione 1130-18-3 C10H15NO2 181.235 乙匹康 3,3-diethyl-5-methyl-1H-pyridine-2,4-dione 467-90-3 C10H15NO2 181.235 —— 1,3,3-triethyl-1H-pyridine-2,4-dione —— C11H17NO2 195.261 —— 3,3-diethyl-5-bromo-1H-pyridine-2,4-dione —— C9H12BrNO2 246.104 —— 1-(2-Bromoethyl)-3,3-diethylpyridine-2,4-dione 131728-22-8 C11H16BrNO2 274.158 —— 3,3-diethyl-5-hydroxymethyl-1H-pyridine-2,4-dione 20096-03-1 C10H15NO3 197.234 3,3-二乙基-1-(4-吗啉基甲基)-2,4(1H,3H)-吡啶二酮 3,3-diethyl-1-morpholin-4-ylmethyl-1H-pyridine-2,4-dione 23192-94-1 C14H22N2O3 266.34
反应信息
-
作为反应物:参考文献:名称:DE930206摘要:公开号:
-
作为产物:参考文献:名称:一种简单的合成方案,用于保护酰胺,内酰胺,尿素和氨基甲酸酯摘要:描述了用三苯甲基(Tr)基保护酰胺,内酰胺,尿素和氨基甲酸酯NH基的新方法。包含其他官能团的分子说明了该方法的实用性。使用三氟乙酸的温和脱保护使其成为在组合合成树脂上连接酰胺基的有用方法。DOI:10.1016/s0040-4039(02)01964-0
文献信息
-
HETEROCYCLIC ANTIVIRAL COMPOUNDS申请人:Li Jim公开号:US20100111900A1公开(公告)日:2010-05-06Compounds having the formula I wherein R 1 , R 2 , R 3a , R 3b , R 3c , R 4 , R 5 and p are as defined herein are Hepatitis C virus NS5b polymerase inhibitors. Also disclosed are compositions and methods for treating an HCV infection and inhibiting HCV replication.
-
New 1-(heterocyclylalkyl)-4-(propionanilido)-4-piperidinyl methyl ester and methylene methyl ether analgesics作者:Jerome R. Bagley、Sheela A. Thomas、Frieda G. Rudo、H. Kenneth Spencer、Brian M. Doorley、Michael H. Ossipov、Thomas P. Jerussi、Mark J. Benvenga、Theodore SpauldingDOI:10.1021/jm00106a051日期:1991.2bulkier aromatic ring systems than the corresponding components of the arylethyl groups of the prototypic methyl ester (carfentanil, 2) and methylene methyl ether (sufentanil, 3 and alfentanil, 4) 4-propionanilido analgesics. Compound 9A (methyl 1-[2-(1H-pyrazol-1-yl)-ethyl]-4-[(1-oxopropyl)phenylamino]-4- piperidinecarboxylate), which exhibited appreciable mu-opioid receptor affinity, was a more potent已经合成了一系列新的1-(杂环烷基)-4-(丙酰胺基)-4-哌啶基甲基酯和亚甲基甲基醚,并进行了药理学评估。在小鼠热板试验中,大多数化合物的镇痛效果(ED50低于1 mg / kg)优于吗啡。这些研究表明,与原型甲基酯(卡芬太尼,2)和亚甲基甲基醚(舒芬太尼,3和阿芬太尼,4)4的芳基乙基的相应组分相比,其结构上更多样化和更庞大的芳环体系具有药理学适应性4-丙酸安宁镇痛药。表现出明显的阿片类受体亲和力的化合物9A(1- [2-(1H-吡唑-1-基)-乙基] -4-[(1-氧丙基)苯基氨基] -4-哌啶羧酸甲酯)强效和短效止痛药,比阿芬太尼具有更少的大鼠呼吸抑制作用。另一方面,邻苯二甲酰亚胺57A和57B对与伤害性传递的介导相关的阿片受体(例如,mu-,kappa-和delta-亚型)表现出微不足道的亲和力,在所有抗伤害感受试验中均显示出镇痛效果。此外,尽管57B与临床阿片类药物相比,在大鼠
-
[EN] TRICYCLIC COMPOUNDS AS VASOPRESSIN V1A RECEPTOR ANTAGONISTS<br/>[FR] COMPOSÉS TRICYCLIQUES UTILISÉS EN TANT QU'ANTAGONISTES DU RÉCEPTEUR DE LA VASOPRESSINE V1A申请人:RICHTER GEDEON NYRT公开号:WO2019116325A1公开(公告)日:2019-06-20The present invention relates to 5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1]benzazepine derivatives of general formula (I) and/or salts thereof and/or geometric isomers thereof and/or stereoisomers thereof and/or enantiomers thereof and/or racemates thereof and/or diastereomers thereof and/or biologically active metabolites thereof and/or prodrugs thereof and/or solvates thereof and/or hydrates thereof and/or polymorphs thereof which are centrally and/or peripherally acting V1a receptor modulators, particularly V1a receptor antagonists. Additional subject of the present invention is the process for the preparation of the compounds and the intermediates of the preparation process as well. The invention also relates to the pharmaceutical compositions containing the compounds or together with one or more other active substances, as well as to the use in the treatment and/or prophylaxis of a disease or condition associated with V1a receptor function.
-
METHODS AND COMPOSITIONS FOR TREATING INFECTION申请人:UNIVERSITY OF ROCHESTER公开号:US20150238473A1公开(公告)日:2015-08-27Provided herein are compositions and methods for treating or preventing infection.本文提供了用于治疗或预防感染的组合物和方法。
-
Structure-Activity Relationship Studies of CNS Agents, Part 22: A Search for New Trazodone-Like Antidepressants: Synthesis and Preliminary Receptor Binding Studies作者:Jerzy L. Mokrosz、Beata Duszynska、Maria H. Paluchowska、S. Charakchieva-Minol、Maria J. MokroszDOI:10.1002/ardp.19953280711日期:——New 1‐phenyl‐ and 1‐(3‐chlorophenyl)piperazines containing a 4‐[3‐(heterocyclic)propyl] fragment were synthesized. It was found that of all the investigated compounds 11b (Ki = 13 ± 2 nM) and 8b (Ki = 38 ± 2 nM) were the most active 5‐HT1A and 5‐HT2A ligands, respectively. Several derivatives (3a, 4a, 8b, 11b, 12b, 13a, and 13b) were selected as good candidates for new, potential antidepressants on
表征谱图
-
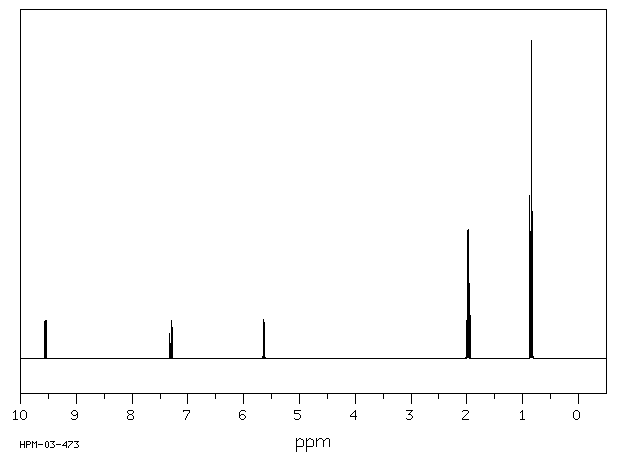
氢谱1HNMR
-
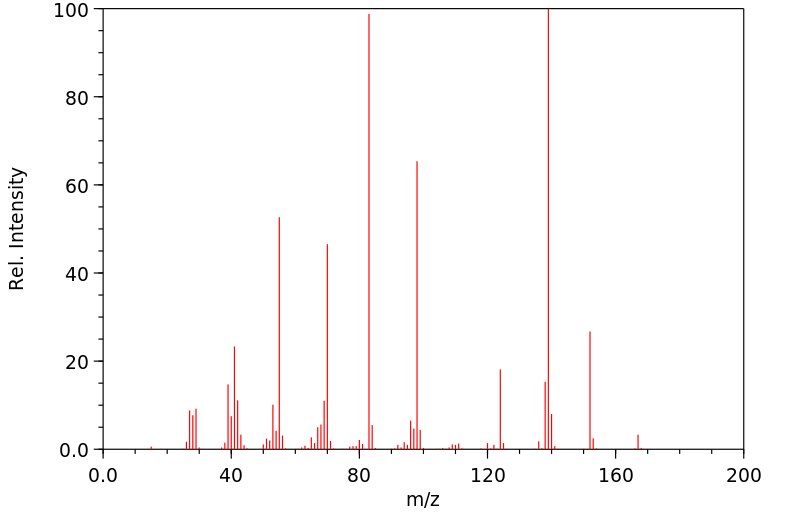
质谱MS
-
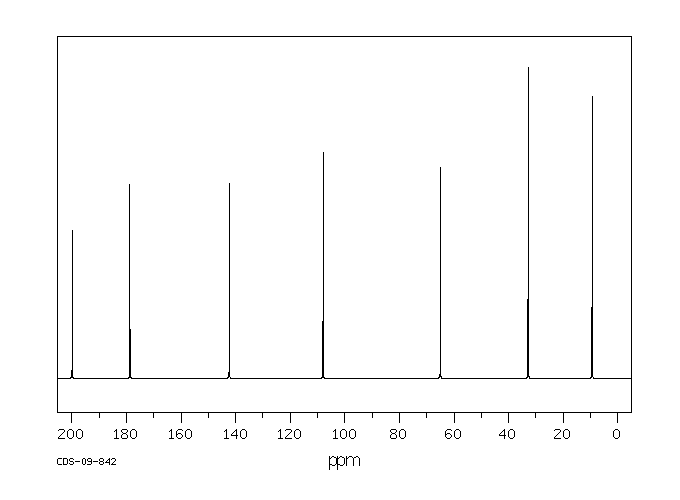
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-