4-(1-(4-hydroxy-3-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-2-methoxyphenol
中文名称
——
中文别名
——
英文名称
4-(1-(4-hydroxy-3-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-2-methoxyphenol
英文别名
4-[1-(4-Hydroxy-3-methoxybenzyl)-1H-benzimidazol-2-yl]-2-methoxyphenol;4-[[2-(4-hydroxy-3-methoxyphenyl)benzimidazol-1-yl]methyl]-2-methoxyphenol
CAS
——
化学式
C22H20N2O4
mdl
MFCD01952969
分子量
376.412
InChiKey
SBVOOAOEAYENPN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:28
-
可旋转键数:5
-
环数:4.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:76.7
-
氢给体数:2
-
氢受体数:5
反应信息
-
作为反应物:描述:二苯氨基甲酰氯 、 4-(1-(4-hydroxy-3-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-2-methoxyphenol 在 吡啶 作用下, 反应 12.0h, 以97%的产率得到参考文献:名称:聚集诱导发射(AIE)活性咪唑的简便合成,用于三氟拉林的灵敏检测摘要:由香草醛,邻苯二胺和N,N-二苯基氨基甲酰氯合成了新型咪唑荧光探针(2)。其结构的特征在于荧光光谱,UV-Vis光谱,1 H NMR,13 C NMR和高分辨率质谱(HRMS)。此外,在THF / MeOH溶液中研究了其聚集诱导的发射(AIE)功能。此外,荧光猝灭实验结果表明,化合物2是潜在的小有机分子荧光探针,对硝基芳香族化合物显示出高选择性和敏感性。此外,该探针可用于三氟拉林的测定,具有快速响应和稳定的特点。探针的荧光响应与三氟拉林的浓度在10至100μM之间表现出良好的线性相关性,并且检出限(LOD)低至5.066μM。最终,该探针成功地用于测定实际样品中三氟拉林的含量,回收率为91.1%至111.2%,表明该探针的适用性和可靠性。DOI:10.1016/j.saa.2021.119880
-
作为产物:描述:N,N'-bis(4-hydroxy-3-methoxybenzylidene)-1,2-phenylenediamine 在 对甲苯磺酸 作用下, 以 neat (no solvent) 为溶剂, 以92%的产率得到4-(1-(4-hydroxy-3-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-2-methoxyphenol参考文献:名称:Facile Synthesis of 1,2-Disubstituted Benzimidazoles Usingp-Toluenesulfonic Acid through Grinding Method摘要:An efficient synthetic method for the highly selective synthesis of pharmacologically active 1,2-disubstituted benzimidazole derivatives fromo-phenylenediamine and various aromatic aldehydes catalyzed byp-toluenesulfonic acid through grinding under solvent-free condition is described. The reaction requires the catalyst only during the conversion of intermediateN,N '-dibenzylidene-o-phenylenediamine into the desired product. The products were obtained within a short time with good yields by using only a mortar and pestle, which makes the proposed method convenient and cost-effective.DOI:10.1134/s1070428020090201
文献信息
-
Chitosan-supported Fe3O4 nanoparticles: a magnetically recyclable heterogeneous nanocatalyst for the syntheses of multifunctional benzimidazoles and benzodiazepines作者:Ali Maleki、Nakisa Ghamari、Maryam KamalzareDOI:10.1039/c3ra47366j日期:——An environmentally benign and clean biopolymer-based heterogeneous nanocatalyst was prepared and its properties and morphology were characterized using scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX) and X-ray diffraction (XRD). Then, its catalytic activity was investigated in two important organic reactions. In these reactions, efficient and selective syntheses of 1,2-disubstituted benzimidazole and 1,5-benzodiazepine derivatives were carried out using 1,2-diamines and aldehydes or ketones in the presence of a catalytic amount of magnetically recoverable chitosan-supported iron oxide nanoparticles (Fe3O4/chitosan), as a biodegradable nanocomposite, in ethanol at ambient temperature in high yields. The Fe3O4/chitosan nanocatalyst can be recovered easily and reused without any significant loss of the catalytic activity.
-
Natural Bio-surfactant for Pseudomulticomponent Synthesis of 2-Aryl-1- aryl Methyl-1H-benzimidazoles作者:Smita T. Morbale、Sachin K. Shinde、Shashikant A. Damate、Madhukar B. Deshmukh、Suresh S. PatilDOI:10.2174/1570178614666170710115331日期:2017.12.11Green chemistry emphasizes the development of environmentally benign chemical processes and technologies. Pseudo-multicomponent synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles using o-phenylenediamine and aromatic aldehydes is carried out by Bronsted acid type bio-surfactant as a catalyst. The green features of this method include the use of biodegradable catalyst obtained from renewable resource i.e. Citrus Limonium extract as bio-surfactant type Bronsted acid, which provides a micellar media for effective cyclocondensation. The critical micellar concentration (cmc) of biosurfactant was determined by conductivity method and visualized by light microscopy measurement. Identity of all pure compounds was ascertained on the basis of FT-IR, 1H NMR and 13C NMR spectroscopic techniques.
-
Nano indium oxide: an efficient catalyst for the synthesis of 1,2-disubstituted benzimidazoles in aqueous media作者:Sougata Santra、Adinath Majee、Alakananda HajraDOI:10.1016/j.tetlet.2012.02.021日期:2012.4Synthesis of 1,2-disubstituted benzimidazoles has been developed by the condensation of diamine with aldehydes using nano In2O3 as an efficient catalyst under mild reaction conditions in aqueous media. The procedure is applicable to aryl, aliphatic, heteroaryl aldehydes. In2O3 nanoparticles are recyclable without the loss of significant catalytic activity.
-
Efficient Synthesis of 2-Aryl-1-arylmethyl-1<i>H</i>-benzimidazoles in Ball Mill without Solvent作者:Meihong Jin、Guowei Song、Zhenjiang Li、Feng Zhou、Bo Fan、Pingkai OuyangDOI:10.1002/jhet.1113日期:2014.112‐Aryl‐1‐arylmethyl‐1H‐benzimidazoles were prepared in excellent yields by the condensation of o‐phenylenediamine with aldehydes under mechanically activated solvent‐free conditions in ball mill using FeCl3·6H2O as the catalyst.
-
A Cu (NO3)2.3H2O catalysed facile synthesis of substituted 4(3H)-quinazolinones and benzimidazoles作者:G A N K DURGAREDDY、R RAVIKUMAR、S RAVI、SRINIVAS R ADAPADOI:10.1007/s12039-012-0343-0日期:2013.1One pot synthesis of alkyl, aryl, heteroaryl mono(2)substituted 4(3H)-quinazolinones and 2-aryl or heteroaryl, 1-arylmethyl or heteroarylmethyl -1H-benzimidazoles using a water soluble Cu (NO3)2.3H2O catalyst at room/ambient temperature in excellent yield. One pot synthesis of alkyl, aryl, heteroaryl mono(2)substituted 4(3H)-quinazolinones and 2-aryl or heteroaryl, 1-arylmethyl or heteroarylmethyl-1H-benzimidazoles
表征谱图
-
氢谱1HNMR
-
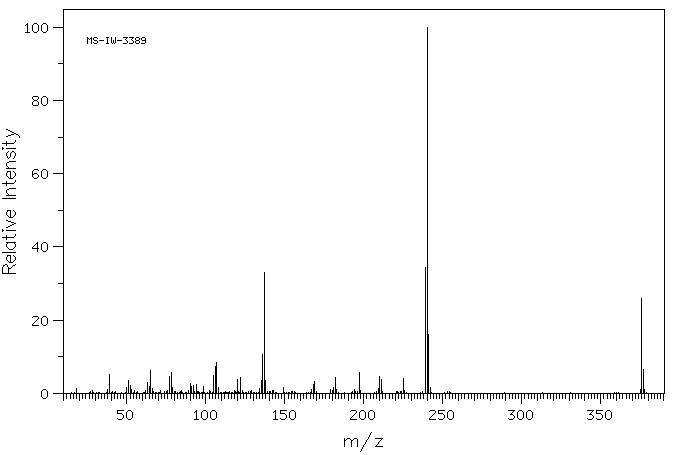
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-(-)-2-(α-(叔丁基)甲胺)-1H-苯并咪唑
(S)-(-)-2-(α-甲基甲胺)-1H-苯并咪唑
麦穗宁
马哌斯汀
颜料橙62
顺式-5,6-二氢-4,5-二甲基-4H-咪唑并[1,5,4-De]喹喔啉
韦罗肟
青菌灵
雷贝拉唑钠
雷贝拉唑硫醚N-氧化物
雷贝拉唑砜 N-氧化物
雷贝拉唑砜
雷贝拉唑杂质2
雷贝拉唑 N-氧化物
雷贝拉唑
阿苯达唑砜
阿苯达唑杂质L
阿苯达唑杂质J(EP)
阿苯达唑杂质J
阿苯达唑杂质F
阿苯达唑杂质14
阿苯达唑杂质13
阿苯达唑亚砜
阿苯达唑
阿苯哒唑砜-D3
阿苯哒唑-D3
阿地本旦
阿司咪唑-d3
阿司咪唑
钠4-[5-氯-2-[(E,3E)-3-[6-氯-1-乙基-3-(4-磺酸丁基)-5-(三氟甲基)苯并咪唑-2-亚基]丙-1-烯基]-3-乙基-6-(三氟甲基)苯并咪唑-1-鎓-1-基]丁烷-1-磺酸盐
邻甲磺酰胺基苯乙酸
那地特罗
达比加群酯杂质M
达比加群酯杂质4
达比加群酯杂质1
达比加群酯杂质
达比加群酯N-氧化物
达比加群酯
达比加群脂杂质10
达比加群甲酯杂质
达比加群杂质J
达比加群杂质J
达比加群杂质F
达比加群杂质E
达比加群杂质D
达比加群杂质C5
达比加群杂质38
达比加群杂质13
达比加群杂质10(DABRC-10)
达比加群杂质10