4-O-beta-吡喃半乳糖基-D-吡喃甘露糖 | 50468-56-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:774.5±60.0 °C(Predicted)
-
密度:1.68±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-5
-
重原子数:23
-
可旋转键数:8
-
环数:1.0
-
sp3杂化的碳原子比例:0.92
-
拓扑面积:197
-
氢给体数:8
-
氢受体数:11
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乳糖 D-(+)-lactose 63-42-3 C12H22O11 342.3 β-乳糖 LACTOSE 5965-66-2 C12H22O11 342.3 D-(+)-纤维二糖 Maltose 133-99-3 C12H22O11 342.3 —— Cellobiose 13360-52-6 C12H22O11 342.3 —— Lactose 63-42-3 C12H22O11 342.3 直链淀粉 maltotriose 9005-82-7 C18H32O16 504.442 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Lactose 63-42-3 C12H22O11 342.3
反应信息
-
作为反应物:描述:、 4-O-beta-吡喃半乳糖基-D-吡喃甘露糖 、 (2S,3R,4S,5R,6R)-2-[(2R,3S,4S,5R)-4,5-dihydroxy-2,5-bis(hydroxymethyl)oxolan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol;hydrate 、 生成 Lactose参考文献:名称:Cellobiose 2-epimerase, its preparation and uses摘要:本发明旨在提供一种热稳定的纤维二糖2-表异构酶及其制备和用途。本发明通过提供一种热稳定的纤维二糖2-表异构酶,编码该酶的DNA,包含该DNA的重组DNA和转化体,生产该酶的方法以及使用该酶制备异构化糖的方法来实现上述目的。公开号:US09175282B2
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 甲醇 、 barium methoxide 作用下, 生成 4-O-beta-吡喃半乳糖基-D-吡喃甘露糖参考文献:名称:Syntheses of Epi-lactose and Lactose摘要:DOI:10.1021/ja01260a031
文献信息
-
Regioselectivity in β-Galactosidase-catalyzed Transglycosylation for the Enzymatic Assembly of<scp>D</scp>-Galactosyl-<scp>D</scp>-mannose作者:Mariko MIYASATO、Katsumi AJISAKADOI:10.1271/bbb.68.2086日期:2004.1different origins. The relative hydrolysis rate of Gal beta-pNP and D-galactosyl-D-mannoses, of various linkages, was also measured in the presence of beta-1,3-galactosidase and was found to correlate well with the ratio of disaccharides formed by transglycosylation. The unexpected regioselectivity using D-mannose can therefore be explained by an anomalous specificity in the hydrolysis reaction. By utilizing
-
Functional reassignment of <i>Cellvibrio vulgaris</i> EpiA to cellobiose 2-epimerase and an evaluation of the biochemical functions of the 4-<i>O</i>-β-<scp>d</scp>-mannosyl-<scp>d</scp>-glucose phosphorylase-like protein, UnkA作者:Wataru Saburi、Yuka Tanaka、Hirohiko Muto、Sota Inoue、Rei Odaka、Mamoru Nishimoto、Motomitsu Kitaoka、Haruhide MoriDOI:10.1080/09168451.2015.1012146日期:2015.6.3
Abstract The aerobic soil bacterium Cellvibrio vulgaris has a β-mannan-degradation gene cluster, including unkA, epiA, man5A, and aga27A. Among these genes, epiA has been assigned to encode an epimerase for converting d-mannose to d-glucose, even though the amino acid sequence of EpiA is similar to that of cellobiose 2-epimerases (CEs). UnkA, whose function currently remains unknown, shows a high sequence identity to 4-O-β-d-mannosyl-d-glucose phosphorylase. In this study, we have investigated CE activity of EpiA and the general characteristics of UnkA using recombinant proteins from Escherichia coli. Recombinant EpiA catalyzed the epimerization of the 2-OH group of sugar residue at the reducing end of cellobiose, lactose, and β-(1→4)-mannobiose in a similar manner to other CEs. Furthermore, the reaction efficiency of EpiA for β-(1→4)-mannobiose was 5.5 × 104-fold higher than it was for d-mannose. Recombinant UnkA phosphorolyzed β-d-mannosyl-(1→4)-d-glucose and specifically utilized d-glucose as an acceptor in the reverse reaction, which indicated that UnkA is a typical 4-O-β-d-mannosyl-d-glucose phosphorylase.
摘要:有氧土壤细菌Cellvibrio vulgaris具有一个β-甘露聚糖降解基因簇,包括unkA、epiA、man5A和aga27A。在这些基因中,epiA被指定为编码一个使d-甘露糖转化为d-葡萄糖的异构酶,尽管EpiA的氨基酸序列与赤霉糖2-异构酶(CEs)的相似。UnkA的功能目前仍未知,显示出与4-O-β-d-甘露基-d-葡萄糖磷酸化酶具有高度相似的序列。在这项研究中,我们使用大肠杆菌的重组蛋白研究了EpiA的CE活性和UnkA的一般特性。重组EpiA催化了赤霉糖、乳糖和β-(1→4)-甘露二糖末端的糖残基的2-OH基的异构化,类似于其他CEs。此外,EpiA对β-(1→4)-甘露二糖的反应效率比对d-甘露高5.5×104倍。重组UnkA对β-d-甘露基-(1→4)-d-葡萄糖进行了磷酸解除作用,并在反向反应中特异地利用d-葡萄糖作为受体,这表明UnkA是一种典型的4-O-β-d-甘露基-d-葡萄糖磷酸化酶。 -
Biochemical Characterization of a Thermophilic Cellobiose 2-Epimerase from a Thermohalophilic Bacterium,<i>Rhodothermus marinus</i>JCM9785作者:Teruyo OJIMA、Wataru SABURI、Hiroki SATO、Takeshi YAMAMOTO、Haruhide MORI、Hirokazu MATSUIDOI:10.1271/bbb.110456日期:2011.11.23Cellobiose 2-epimerase (CE) reversibly converts glucose residue to mannose residue at the reducing end of β-1,4-linked oligosaccharides. It efficiently produces epilactose carrying prebiotic properties from lactose, but the utilization of known CEs is limited due to thermolability. We focused on thermoholophilic Rhodothermus marinus JCM9785 as a CE producer, since a CE-like gene was found in the genome of R. marinus DSM4252. CE activity was detected in the cell extract of R. marinus JCM9785. The deduced amino acid sequence of the CE gene from R. marinus JCM9785 (RmCE) was 94.2% identical to that from R. marinus DSM4252. The N-terminal amino acid sequence and tryptic peptide masses of the native enzyme matched those of RmCE. The recombinant RmCE was most active at 80 °C at pH 6.3, and stable in a range of pH 3.2–10.8 and below 80 °C. In contrast to other CEs, RmCE demonstrated higher preference for lactose over cellobiose.Cellobiose 2-epimerase(CE)可逆地将β-1,4-连接寡糖还原端的葡萄糖残基转化为甘露糖残基。它能有效地从乳糖中产生具有益生特性的表乳糖,但由于热稳定性的原因,对已知 CE 的利用受到了限制。由于在R. marinus DSM4252的基因组中发现了一个类似CE的基因,因此我们重点研究了嗜热型Rhodothermus marinus JCM9785作为CE生产者的情况。在 R. marinus JCM9785 的细胞提取物中检测到了 CE 活性。R. marinus JCM9785的CE基因(RmCE)的氨基酸序列与R. marinus DSM4252的氨基酸序列有94.2%的相同度。原生酶的 N 端氨基酸序列和胰蛋白酶肽的质量与 RmCE 相吻合。重组的 RmCE 在 pH 值为 6.3、温度为 80 ℃ 时活性最高,在 pH 值为 3.2-10.8 和低于 80 ℃ 的范围内稳定。与其他 CE 相比,RmCE 对乳糖的偏好高于纤维生物糖。
-
CELLOBIOSE 2-EPIMERASE, ITS PREPARATION AND USES申请人:Watanabe Hikaru公开号:US20120040407A1公开(公告)日:2012-02-16The present invention has objects to provide a thermostable cellobiose 2-epimerase, its preparation and uses. The present invention attains the above objects by providing a thermostable cellobiose 2-epimerase, a DNA encoding the enzyme, a recombinant DNA and transformant comprising the DNA, a process for producing the enzyme, and a process for producing isomerized saccharides using the enzyme.
-
Haworth et al., Journal of the Chemical Society, 1930, p. 2636,2638作者:Haworth et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
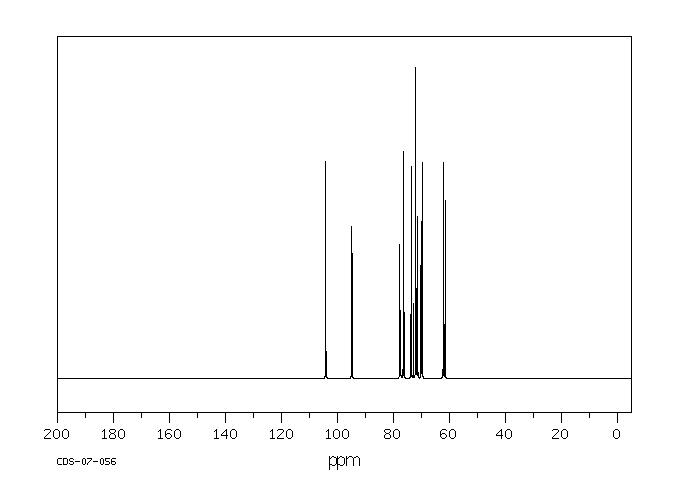
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息