二乙基砜 | 597-35-3
中文名称
二乙基砜
中文别名
1,1’-磺酰基二乙烷;乙基砜;二乙砜;二乙基砜 [597-35-3]
英文名称
diethylsulfone
英文别名
diethyl sulphone;(ethylsulfonyl)ethane;Diethyl sulfone;diethyl sulfate;ethyl sulfone;1-ethylsulfonylethane
CAS
597-35-3
化学式
C4H10O2S
mdl
MFCD00007569
分子量
122.188
InChiKey
MBDUIEKYVPVZJH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:73-74 °C(lit.)
-
沸点:246 °C755 mm Hg(lit.)
-
密度:1.357
-
闪点:246°C
-
溶解度:可溶于氯仿、乙酸乙酯、甲醇(少量)
计算性质
-
辛醇/水分配系数(LogP):-0.6
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:42.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S22,S24/25
-
WGK Germany:3
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:| 室温 干燥 |
SDS
| Name: | Ethyl sulfone 99+% Material Safety Data Sheet |
| Synonym: | Diethyl sulphon |
| CAS: | 597-35-3 |
Synonym:Diethyl sulphon
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 597-35-3 | Ethyl sulfone | 99+ | 209-898-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 597-35-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: very slightly yellow
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 246 deg C @ 755.00mm Hg
Freezing/Melting Point: 73.00 - 74.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: C4H10O2S
Molecular Weight: 122.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 597-35-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Ethyl sulfone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 597-35-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 597-35-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 597-35-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途:用作有机合成中的中间体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙亚磺酰乙烷 diethyl sulphide 70-29-1 C4H10OS 106.189 2-(乙烷磺酰)乙醇 2-ethanesulfonylethanol 513-12-2 C4H10O3S 138.188 乙基乙烯基砜 ethyl vinyl sulfone 1889-59-4 C4H8O2S 120.172 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-氯-2-(乙基磺酰基)乙烷 2-chloroethyl ethyl sulfone 25027-40-1 C4H9ClO2S 156.633 二异丙基砜 diisopropyl sulfone 595-50-6 C6H14O2S 150.242 芥菜砜 bis(2-chloroethyl)sulfone 471-03-4 C4H8Cl2O2S 191.078
反应信息
-
作为反应物:参考文献:名称:磺酰氯直接对烷基砜进行离子氯化摘要:Cl原子的引入通常发生在烷基砜的α原子的α位。在本文中,描述了一种新的烷基砜与磺酰氯的离子氯化方法,其中最值得注意的观察是二乙基砜或环丁砜(四氢噻吩-1,1-二氧化物)的独家或高度选择性的β-氯化。这种方法的最成功的合成应用程序是通过例举7-硫杂二环[2.2.1]庚烷-7,7-二氧化物的氯化,这提供2-外-和2-内型-氯-7-硫杂二环[2.2.1 ]庚烷-7,7-二氧化物,这是可能是因为不希望的均裂SO的难以通过自由基氯化获得,2 C键裂变。2-外将如此获得的-氯-7-噻二环[2.2.1]庚烷-7,7-二氧化物选择性还原,得到2-外-氯-7-硫代双环[2.2.1]庚烷。还讨论了这种氯化的机理。DOI:10.1016/s0040-4020(01)97262-x
-
作为产物:参考文献:名称:Palladium Catalyzed Hydrogenation of .alpha.,.beta.-Unsaturated Sulfones and Phosphonates摘要:The binuclear palladium complex, [(Bu(2)(t)PH)-PdPBu(2)(t)](2), when treated with oxygen, catalyzes the hydrogenation of the double bond of alpha,beta-unsaturated sulfones and phosphonates in THF at room temperature and 1 atm of hydrogen pressure. Saturated sulfones and phosphonates were isolated in 49-93% yields.DOI:10.1021/jo00094a001
-
作为试剂:描述:3-苯丙烯溴酸酯 在 aluminum (III) chloride 、 甲烷磺酸 、 二乙基砜 、 potassium tert-butylate 、 一水合肼 作用下, 以 N,N-二甲基乙酰胺 、 二乙二醇 为溶剂, 反应 49.0h, 生成 苯并[a]蒽参考文献:名称:统一 13C 标记的多环芳烃的合成†摘要:设计了收敛合成途径,用于从 U- 13 C-苯和其他简单的市售13 C 起始化合物有效地从头合成一系列均匀13 C标记的多环芳烃。所有目标产物均以优异的收率获得,包括交替PAH U- 13 C-萘、U- 13 C-菲、U- 13 C-蒽、U- 13 C-苯并[ a ]蒽、U- 13 C-芘和非交替PAH U- 13 C-荧蒽。DOI:10.1039/c0ob01107j
文献信息
-
Oxidation of Organic Compounds by Potassium Permanganate Supported on Montmorillonite K10作者:Ahmad Shaabani、Ayoob Bazgir、Donald G. LeeDOI:10.1081/scc-200031039日期:2004.1.1Abstract The oxidation of organic compounds by potassium permanganate supported on Montmorillonite K10 has been studied under solvent‐free conditions and the results compared with those from corresponding reactions where the reductants are dissolved in methylene chloride. Under both sets of conditions, primary benzylic and secondary alcohols are converted to aldehydes and ketones respectively, sulfides
-
Controlled α-mono- and α,α-di-halogenation of alkyl sulfones using reagent–solvent halogen bonding作者:Christopher M. Poteat、Vincent N. G. LindsayDOI:10.1039/c9cc00550a日期:——The direct and selective α-mono-bromination of alkyl sulfones was achieved through base-mediated electrophilic halogenation. The appropriate combination of solvent and electrophilic bromine source was found to be critical to control the nature of the products formed, where reagent–solvent halogen bonding is proposed to control the selectivity via alteration of the effective size of the electrophilic
-
Mono- and dinuclear complexes of sulfones with the tetrachlorides of group 4作者:Paolo Biagini、Fausto Calderazzo、Fabio Marchetti、Guido Pampaloni、Stefano Ramello、Mario Salvalaggio、Roberto Santi、Silvia SperaDOI:10.1039/b405871b日期:——The reactions of dialkyl sulfones [R2SO2: R = Me (a), Et (b), Ph (c), R2 = –(CH2)4– (d)] with the metal tetrachlorides of Group 4 [MCl4: M = Ti (1), Zr(2), Hf (3)] give different products mainly depending on the sulfone/M molar ratio. Compounds of formula [M2Cl8(R2SO2)2] [M = Ti, R2 = –(CH2)4– (1d); M = Zr, R = Et (2b), R = Ph (2c)] and [MCl4(R2SO2)2] (sulfone/M = 2) [M = Ti, R = Me (1aa); M = Zr,二烷基砜[R 2 SO 2:R = Me(a),Et(b),Ph(c),R 2 =-(CH 2)4-(d)]与第4族金属四氯化物的反应MCl 4:M = Ti(1),Zr(2),Hf(3)]给出不同的产物,主要取决于砜/ M摩尔比。式[M 2 Cl 8(R 2 SO 2)2 ]的化合物[M = Ti,R 2 =-(CH 2)4-(1d);M = Zr,R = Et(2b),R = Ph(2c)]和[MCl 4(R 2 SO 2)2 ](砜/ M = 2)[M = Ti,R = Me(1aa);M = Zr,R = Me(2aa),R = Ph(2cc),R 2 = –(CH 2)4 –(2dd);M = Hf,R = Me(3aa),R 2 = –(CH 2)4 –(3dd)]。经过X射线衍射方法双核钛和锆加合物,钛[Ti 2氯8(μ-sulfolane- Ö,ö ')2 ](图1D)和[Zr的2氯8(μ-PH
-
Fe<sub>3</sub>O<sub>4</sub>/PEG-SO<sub>3</sub>H as a heterogeneous and magnetically-recyclable nanocatalyst for the oxidation of sulfides to sulfones or sulfoxides作者:Saeideh Mirfakhraei、Malak Hekmati、Fereshteh Hosseini Eshbala、Hojat VeisiDOI:10.1039/c7nj02513k日期:——glycol-coated Fe3O4 nanocomposite (Fe3O4/PEG-SO3H) as a greatly effective and ecological nanocatalyst for the selective oxidation of sulfides to sulfoxides or sulfones with brilliant yields under solvent-free conditions by employing 30% hydrogen peroxide as the oxidant. A number of sulfides containing alcohol, ester, and aldehyde functional groups were fruitfully and selectively oxidized without altering the desired
-
Cellulose supported manganese dioxide nanosheet catalyzed aerobic oxidation of organic compounds作者:Ahmad Shaabani、Zeinab Hezarkhani、Shabnam ShaabaniDOI:10.1039/c4ra11101j日期:——Cellulose supported manganese dioxide nanosheets, as a heterogeneous bio-supported and green catalyst, were synthesized by soaking porous cellulose in a potassium permanganate solution. The prepared catalyst was used effectively for the oxidation of various types of alkyl arenes, alcohols and sulfides to their corresponding carbonyl and sulfoxide compounds, respectively in high yields using air as
表征谱图
-
氢谱1HNMR
-
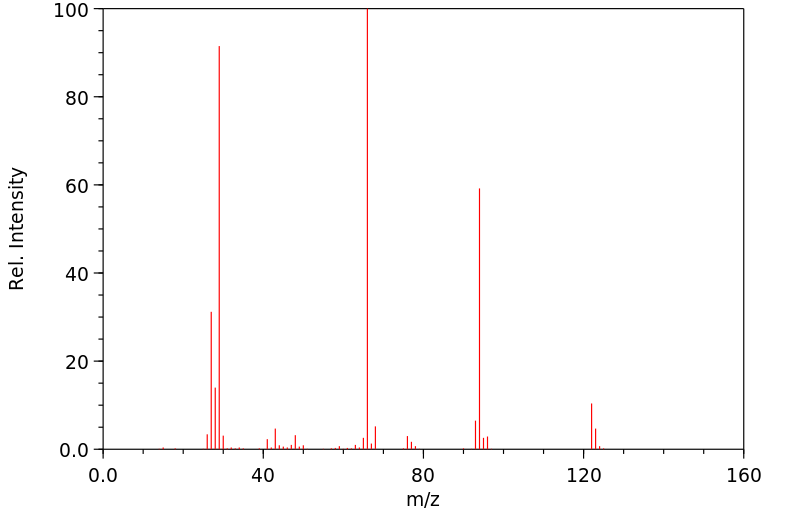
质谱MS
-
碳谱13CNMR
-
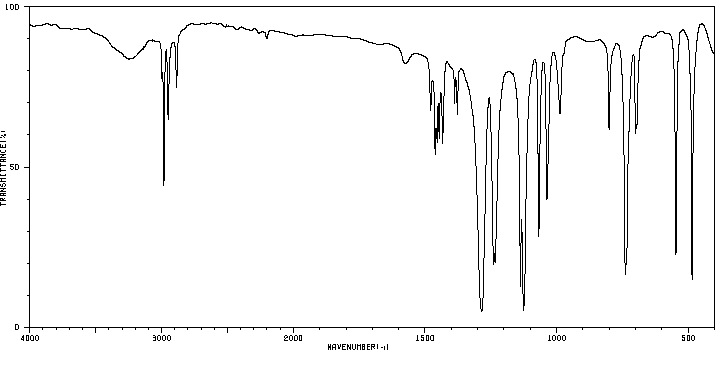
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
辛基甲烷硫代磺酸酯
辛基二砜
蚜灭多砜
蔊菜素
羟基十四烷磺酸钠
糖芥灵
磺酰基二乙睛
磺酰基二[三溴甲烷]
硫酸丙酯
硫酰二丙腈(SDPN)
硫甲磺酸钠
硫氰酸硫酯
硫杂环丁烷-3-羧酸1,1-二氧化物
硫杂环丁烷-1,1-二氧化物
砜吸磷
甲磺酰乙酸甲酯
甲磺酰乙酸
甲磺酰乙酮
甲烷磺酰基氰化物
甲烷磺酰基叠氮化物
甲烷磺酰基乙酸乙酯
甲烷硫代磺酸戊酯
甲烷硫代磺酸丁酯
甲烷硫代磺酸S-(三氯乙烯基)酯
甲烷硫代磺酸 S-(2-羟基乙基-1,1,2,2-D4)酯
甲基磺酰基甲胺
甲基磺酰基甲基磺酰基甲烷
甲基磺酰基甲基二硫基甲基磺酰基甲烷
甲基磺酰乙腈
甲基硫代磺酸甲酯
甲基癸基砜
甲基乙烯砜
甲基乙基砜
甲基3-(乙基磺酰基)丙酸酯
甲基-三聚乙二醇-砜-四聚乙二醇-炔基
环戊基磺酰基环己烷
环己烷,[[(三氟甲基)磺酰]乙炔基]-
环己基三氟甲基砜
环丙胺,N-[2-(2,4,5-三甲基苯氧基)乙基]-
特丁硫磷氧砜
烯丙基二甲基砜
炔基-四聚乙二醇-SULFONE-四聚乙二醇-羧酸
炔基-三聚乙二醇-SULFONE-三聚乙二醇-炔基
溴甲基甲烷硫代磺酸酯
涕灭砜威
氯甲基叔丁基砜
氯甲基三氯甲基砜
氯(甲磺酰基)甲烷
氯(甲磺酰基)乙烷
氯(氯甲基磺酰基)甲烷