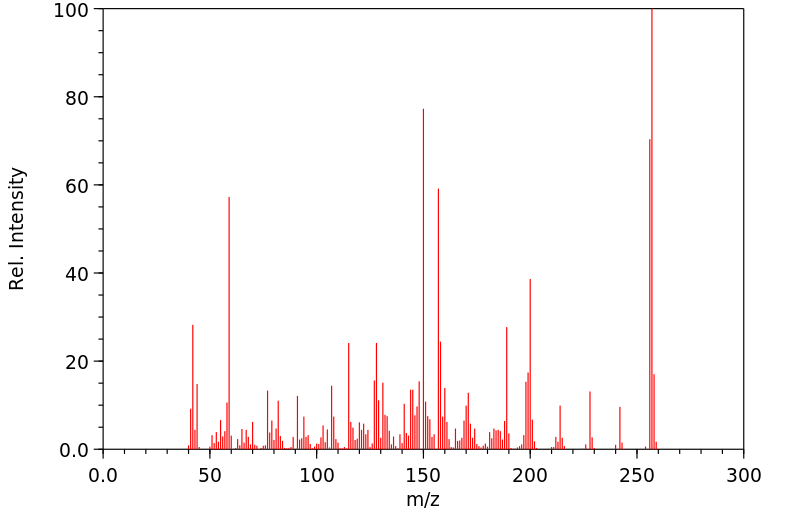
代谢
右奥芬是右美沙芬的一个已知人体代谢物。
Dextrorphan is a known human metabolite of dextromethorphan.
来源:NORMAN Suspect List Exchange