(苯甲酰基氨基)硫脲 | 5351-66-6
中文名称
(苯甲酰基氨基)硫脲
中文别名
1-苯甲酰氨基硫脲
英文名称
benzoyl thiosemicarbazide
英文别名
1-benzoyl-thiosemicarbazide;2-benzoylhydrazinecarbothioamide;2-benzoylhydrazine-1-carbothioamide;1-benzoyl-3-thiosemicarbazide;1-Benzoyl-thiosemicarbazid;1-Benzoyl-3-thiosemicarbazid;benzamidothiourea
CAS
5351-66-6
化学式
C8H9N3OS
mdl
MFCD00086630
分子量
195.245
InChiKey
OWKWAVBMKKZMHE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:199-200 °C
-
密度:1.325±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:99.2
-
氢给体数:3
-
氢受体数:2
安全信息
-
海关编码:2930909090
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:设计和开发1,3,5-三嗪-噻二唑杂化物作为有效的腺苷A2A受体(A2AR)拮抗剂,可改善帕金森氏病。摘要:各种研究表明,腺苷A 2 A受体(A 2 ARs)拮抗剂可通过改善多巴胺的传播而在帕金森病(PD)中具有深远的治疗功效,因此在逆转运动缺陷和与该疾病相关的锥体外系症状方面具有活性。 因此,在本研究中,我们显示了新型的1,3,5-三嗪-噻二唑衍生物作为有效的A 2 AARs拮抗剂的发展。在放射性配体结合测定中,与A 1 R相比,这些分子显示出与A 2 AR的优异结合亲和力,并且具有显着的选择性。结果表明,化合物7e是测试系列中最有效的A 2 AR拮抗剂。在与A 2 AR蛋白模型对接分析中,化合物7e通过与His264,Tyr271,His278,Glu169,Ala63,Val84,Ile274,Met270和Phe169进行多次原子间接触,发现其被深深地埋入衬里的受体腔中。总的来说,我们的研究证明了1,3,5-三嗪-噻二唑杂合体是设计新型A 2 A拮抗剂的高效支架。DOI:10.1016/j.neulet.2020.135222
-
作为产物:描述:参考文献:名称:鉴定1,2,4-三唑为新型胸苷磷酸化酶抑制剂:未来的抗肿瘤药物。摘要:胸苷磷酸化酶(TP)在几种实体瘤中过度表达,其抑制作用可提供适用于癌症药物发现的独特靶标。已经以高产率合成了一系列1,2,4-三唑3a-3l,随后评估了合成的三唑3a-3l对胸苷磷酸化酶的抑制潜力。在这十二个类似物中,五个类似物3b,3c,3f,3l和3l对胸苷磷酸化酶表现出良好的抑制潜力。以IC50值表示的抑制潜力在61.98±0.43至273.43±0.96μM范围内,将7-地塞黄嘌呤用作标准抑制剂,IC50 = 38.68±4.42μM。这些结果的鼓励下,合成了更多的类似物1,2,4-三唑-3-巯基羧酸4a-4g,并评估了它们对胸苷磷酸化酶的抑制潜力。在该系列中,六个类似物4b-4g在43.86±1.11-163.43±2.03μM范围内表现出良好的抑制潜力。1,2,4-三唑酸4d的血管生成反应使用鸡绒毛膜尿囊膜(CAM)分析进行了评估。根据这些发现,讨论了选定的三唑的结构活性关系和DOI:10.1016/j.bioorg.2019.01.005
文献信息
-
Synthesis and Antimicrobial Evaluation of Some New 4,5′-Bisthiazoles作者:Tibor Rozsa、Mihaela Duma、Laurian Vlase、Ioana Ionuţ、Adrian Pîrnău、Brînduşa Tiperciuc、Ovidiu OnigaDOI:10.1002/jhet.2054日期:2015.7bisthiazole derivatives represent a prevalent scaffold in the antimicrobial drug discovery. Therefore, we have decided to synthesize some new series of 4,5′‐bisthiazoles. A total of 17 compounds were synthesized, their structural elucidation being based on elemental analysis (C,H,N,S) and spectroscopic data (MS and 1H NMR). Their in vitro antimicrobial activities were assessed against several Gram‐positive
-
1,3-Cycloaddition of cyclic isothioureas to heterocumulenes and fungitoxicity of the resulting 1,2,4-triazolo(3,4-c)-1,2,4-dithiazoles.作者:H. SINGH、L. D. S. YADAV、K. S. SHARMADOI:10.1271/bbb1961.47.1017日期:——Phenacylation of 5-aryl-3-mercapto-1, 2, 4-triazoles (I) furnished 5-aryl-3-phenacylthio-1, 2, 4-triazoles (II) which reacted with CS2 and aryl isothiocyanates to give 5-aryl-1, 2, 4-triazolo[3, 4-c]-1, 2, 4-dithiazole-3-thiones (III) and 5-aryl-3-arylimino-l, 2, 4-triazolo[3, 4-c]-1, 2, 4-dithiazoles (IV), respectively. (IV) on refluxing with CS2 yielded (III) which, when heated with aryl isothiocyanates, regenerated (IV). Compounds (II)-(IV) were compared with Dithane M-45 for their fungitoxicity against Helminthosporium oryzae and Fusarium oxysporium. The screening results have been correlated with the structural features of the tested compounds.
-
Development of phenyltriazole thiol-based derivatives as highly potent inhibitors of DCN1-UBC12 interaction作者:Wenjuan Zhou、Chenhao Xu、Guanjun Dong、Hui Qiao、Jing Yang、Hongmin Liu、Lina Ding、Kai Sun、Wen ZhaoDOI:10.1016/j.ejmech.2021.113326日期:2021.5Defective in cullin neddylation 1(DCN1) is a co-E3 ligase that is important for cullin neddylation. Dysregulation of DCN1 highly correlates with the development of various cancers. Herein, from the initial high-throughput screening, a novel hit compound 5a containing a phenyltriazole thiol core (IC50 value of 0.95 μM for DCN1-UBC12 interaction) was discovered. Further structure-based optimization leadscullin neddylation 1(DCN1)中的缺陷是co-E3连接酶,对cullin neddylation很重要。DCN1的失调与各种癌症的发生高度相关。在此,从最初的高通量筛选中,发现了含有苯基三唑硫醇核(对于DCN1-UBC12相互作用的IC 50值为0.95μM)的新型命中化合物5a。进一步的基于结构的优化导致了SK-464的发展(IC 50值为26 nM)。我们发现SK-464不仅在体外直接与DCN1结合,但也参与细胞DCN1,抑制cullin3的二联化,并阻碍了两个DCN1过表达的鳞状癌细胞系(KYSE70和H2170)的迁移和侵袭。这些发现表明,SK-464可能是靶向DCN1-UBC12相互作用的新型先导化合物。
-
Synthesis and in vitro Evaluation of West Nile Virus Protease Inhibitors Based on the 2-{6-[2-(5-Phenyl-4<i>H</i>-{1,2,4]triazol-3-ylsulfanyl)acetylamino]benzothiazol-2-ylsulfanyl}acetamide Scaffold作者:Sanjay Samanta、Ting Liang Lim、Yulin LamDOI:10.1002/cmdc.201300114日期:2013.6NS2B‐NS3 protease inhibitor with a 2‐6‐[2‐(5‐phenyl‐4H‐[1,2,4]triazol‐3‐ylsulfanyl)acetylamino]benzothiazol‐2‐ylsulfanyl}acetamide scaffold was identified during screening. Optimization of this initial hit by synthesis and screening of a focused compound library with this scaffold led to the identification of a novel uncompetitive inhibitor (1 a24, IC50=3.4±0.2 μM) of the WNV NS2B‐NS3 protease. Molecular近年来,由西尼罗河病毒(WNV)感染引起的临床症状严重程度恶化,老年人神经侵袭性疾病的发生频率增加。由于目前尚无成功的抗WNV疗法用于人类,因此迫切需要为开发新的针对该病毒的化学疗法而不断努力。由于其在病毒复制中的重要性及其独特的底物偏爱性,病毒NS2B-NS3蛋白酶是有希望的病毒抑制靶标。在这项研究中,WNV NS2B- 蛋白酶抑制剂具有2- 6- [2-(5-苯基-4H]在筛选过程中发现了[[1,2,4]三唑-3-基硫烷基]乙酰氨基]苯并噻唑-2-基硫基}乙酰胺支架。通过合成和的筛选该初始命中的优化聚焦化合物库与此支架导致的新颖非竞争性抑制剂的鉴定(1 A24,IC 50 = 3.4±0.2μ中号的WNV的NS2B- 蛋白酶)。1 a24分子对接至WNV蛋白酶表明该化合物干扰NS2B辅因子与 蛋白酶的生产性相互作用,并且是WNV 蛋白酶的变构抑制剂。
-
Synthesis and antifungal activity of novel 3,6-diaryl-5<i>H</i>-[1,2,4]triazolo[4,3-<i>b</i>][1,2,4]triazepines作者:Monika GuptaDOI:10.1002/jhet.5570440508日期:2007.9presence of catalytic amount of p-TsOH and N,N-dimethylformamide as an energy transfer medium under microwave irradiation and as solvent with oil-bath heating at 80 °C affords novel 3,6-diaryl-5H-[1,2,4]triazolo[4,3-b]-1,2,4]triazepines 8. The structures of the synthesized compounds were established on the basis of 1H NMR, IR, mass spectral data and elemental analysis.
表征谱图
-
氢谱1HNMR
-
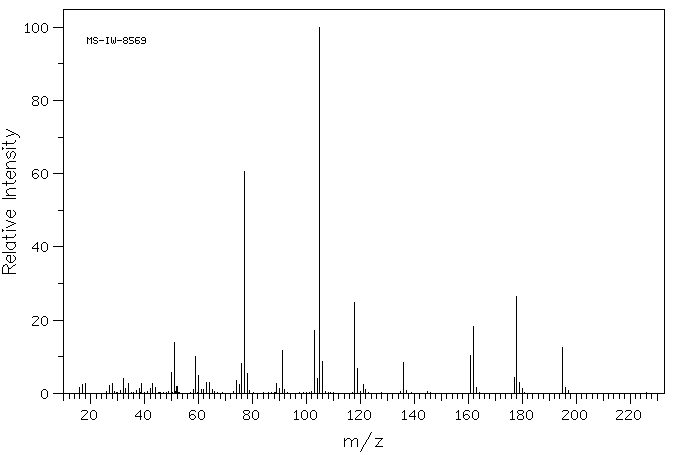
质谱MS
-
碳谱13CNMR
-
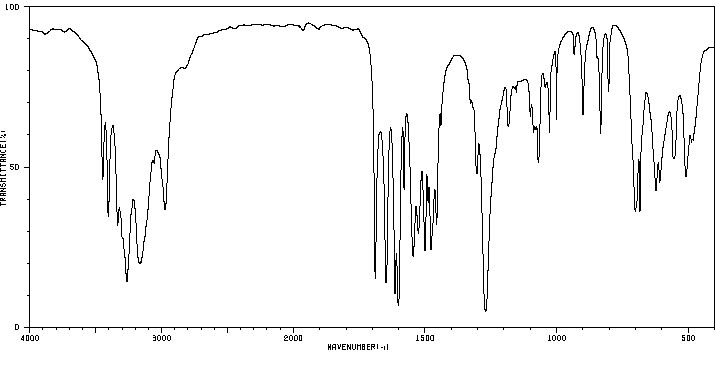
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫