1-硝基-4-亚硝基苯 | 4485-08-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:75.2
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2904209090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对二硝基苯 para-dinitrobenzene 100-25-4 C6H4N2O4 168.109 4-硝基苯基羟胺 4-Nitro-N-phenylhydroxylamine 16169-16-7 C6H6N2O3 154.125 4-硝基苯胺 4-nitro-aniline 100-01-6 C6H6N2O2 138.126 —— 4-nitrophenyl azide 1516-60-5 C6H4N4O2 164.123 —— S,S-dimethyl-N-(p-nitrophenyl)sulfilimine 27691-52-7 C8H10N2O2S 198.246 —— N-(4-nitro-phenyl)-N-nitroso-hydroxylamine 5180-42-7 C6H5N3O4 183.123 —— (4-Nitroanilino) 2,2-dimethylpropanoate 88867-70-3 C11H14N2O4 238.243 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 对二硝基苯 para-dinitrobenzene 100-25-4 C6H4N2O4 168.109 4-硝基苯基羟胺 4-Nitro-N-phenylhydroxylamine 16169-16-7 C6H6N2O3 154.125 N-甲基对硝基苯胺 N-methyl(p-nitroaniline) 100-15-2 C7H8N2O2 152.153 —— 4-nitrophenyl azide 1516-60-5 C6H4N4O2 164.123 二(4-硝基苯基)二氮烯 bis(4-nitrophenyl)diazene 89103-79-7 C12H8N4O4 272.22 —— trans-4-nitroazobenzene 20488-61-3 C12H9N3O2 227.222 —— disperse orange 3 70734-98-4 C12H10N4O2 242.237 对硝基偶氮苯 4-nitroazobenzene 2491-52-3 C12H9N3O2 227.222 —— 1,2-bis(4-nitrophenyl)diazene 3646-57-9 C12H8N4O4 272.22 —— N-(4-nitro-phenyl)-N-nitroso-hydroxylamine 5180-42-7 C6H5N3O4 183.123 —— 1-(4-nitrophenyl)-2-bromodiazene 1-oxide 172265-51-9 C6H4BrN3O3 246.02 —— 4-Nitro-4'-dimethylamino-diphenylamin 5235-63-2 C14H15N3O2 257.292 —— (E)-1-(4-Fluorophenyl)-2-(4-nitrophenyl)diazene 51788-98-8 C12H8FN3O2 245.213 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Kinetics of the oxidation of aromatic C-nitroso compounds by nitrogen dioxide摘要:对二氧化氮在四氯化碳中氧化亚硝基苯的过程进行了重新研究,反应速率与亚硝基苯和二氧化氮的浓度呈一阶依赖关系,反应物的化学计量为 1∶1。研究发现,反应速率对微量水的存在非常敏感,因此开发了溶剂的强化干燥程序,以便将反应速率降至恒定水平。对于亚硝基苯和一系列取代的亚硝基苯,采用停流技术和二氧化氮过量的假一阶条件,对其在四氯化碳中的氧化反应动力学进行了研究,温度范围为 291-313K。获得了 15 个亚硝基烯烃的阿伦尼斯参数,并参考哈米特 σ 常数和通过 CNDO 计算确定的亚硝基氮上的电子群讨论了所获得的结果。结论是该反应是通过亚硝基氮原子的自由基加成机制发生的,生成不稳定的氨基氧中间体,该中间体迅速分解,生成相应的硝基苯和一氧化氮。DOI:10.1039/a700258k
-
作为产物:参考文献:名称:Solvatochromism and acidochromism of azobenzene-functionalized poly(vinyl amines)摘要:多乙烯胺(PVAm)对氟芳香化合物的亲核取代反应是一种制备多种偶氮苯功能化PVAms的适宜方法。氟芳香反应剂在水中的溶解度通过与2,6-O-二甲基-β-环糊精(DMCD)络合得以调节。功能化程度已通过参考体系中的模型化合物确定。这些聚合物和模型化合物的溶剂化变色性质已通过Kamlet-Taft和Catalán的成熟线性溶剂化能关系(LSER)进行了研究并得到了解释。溶剂化变色行为中最主要的影响来自于与不同偶极性/极化率的溶剂的相互作用。同时,溶剂的碱性也被认为在偶氮苯功能化PVAms的溶剂化变色中起着重要作用,这显示了聚合物链的影响。此外,随着介质酸性的增强,UV/可见吸收光谱显示出向红移的特征。这特别突出了PVAm骨架对所连接的生色团颜色的影响。DOI:10.1039/c2nj40313g
-
作为试剂:描述:参考文献:名称:亚硝基烯反应中的羟基方向性:手性烯丙醇的非对映和区域选择性胺化摘要:DOI:10.1021/ja001752w
文献信息
-
Optical control of GIRK channels using visible light作者:Julie B. Trads、Jessica Burgstaller、Laura Laprell、David B. Konrad、Luis de la Osa de la Rosa、C. David Weaver、Herwig Baier、Dirk Trauner、David M. BarberDOI:10.1039/c6ob02153k日期:——G-protein coupled inwardly rectifying potassium (GIRK) channels are an integral part of inhibitory signal transduction pathways, reducing the activity of excitable cells via hyperpolarization. They play crucial roles in processes such as cardiac output, cognition and the coordination of movement. Therefore, the precision control of GIRK channels is of critical importance. Here, we describe the development
-
Synthesis of Azoxybenzenes by Reductive Dimerization of Nitrosobenzene作者:Yu-Feng Chen、Jing Chen、Li-Jen Lin、Gary Jing ChuangDOI:10.1021/acs.joc.7b01887日期:2017.11.3Herein we report an effective and simple preparation method of substituted azoxybenzenes by reductive dimerization of nitrosobenzenes. This procedure requires no additional catalyst/reagent and can be applied to substrates with a wide range of substitution patterns.在本文中,我们报道了通过亚硝基苯的还原二聚作用制备取代的乙氧基苯的有效而简单的方法。该程序不需要额外的催化剂/试剂,并且可以应用于具有广泛取代模式的底物。
-
Azobenzene-based inhibitors of human carbonic anhydrase II作者:Leander Simon Runtsch、David Michael Barber、Peter Mayer、Michael Groll、Dirk Trauner、Johannes BroichhagenDOI:10.3762/bjoc.11.127日期:——
Aryl sulfonamides are a widely used drug class for the inhibition of carbonic anhydrases. In the context of our program of photochromic pharmacophores we were interested in the exploration of azobenzene-containing sulfonamides to block the catalytic activity of human carbonic anhydrase II (hCAII). Herein, we report the synthesis and in vitro evaluation of a small library of nine photochromic sulfonamides towards hCAII. All molecules are azobenzene-4-sulfonamides, which are substituted by different functional groups in the 4´-position and were characterized by X-ray crystallography. We aimed to investigate the influence of electron-donating or electron-withdrawing substituents on the inhibitory constant
K i. With the aid of an hCAII crystal structure bound to one of the synthesized azobenzenes, we found that the electronic structure does not strongly affect inhibition. Taken together, all compounds are strong blockers of hCAII withK i = 25–65 nM that are potentially photochromic and thus combine studies from chemical synthesis, crystallography and enzyme kinetics.芳基磺胺是一类广泛使用的药物,用于抑制碳酸酐酶。在我们的光致变色药物基团项目中,我们对含有偶氮苯基磺胺的化合物进行了探索,以阻断人类碳酸酐酶II(hCAII)的催化活性。在这里,我们报告了对九种光致变色磺胺化合物进行合成和体外评价,以用于hCAII。所有分子均为偶氮苯-4-磺胺,其在4´-位置被不同的功能基团取代,并通过X射线晶体学进行表征。我们旨在研究电子给体或电子吸引基团对抑制常数Ki的影响。通过结合合成的偶氮苯之一与hCAII晶体结构结合的结构,我们发现电子结构并不强烈影响抑制作用。总的来说,所有化合物都是hCAII的强效阻断剂,Ki = 25-65 nM,可能是光致变色的,因此结合了化学合成、晶体学和酶动力学研究。 -
N-Arylhydroxamic Acids as Novel Oxidoreductase Substrates作者:Juozas Kulys、Heinz-Josef Deussen、Kastis Krikstopaitis、Rikke Lolck、Palle Schneider、Arturas ZiemysDOI:10.1002/1099-0690(200109)2001:18<3475::aid-ejoc3475>3.0.co;2-k日期:2001.9N-Arylhydroxamic acids (AHAs) are promising novel N−OH mediators for oxidoreductase catalysis. They are electrochemically active compounds with a redox potential of 0.31−0.41 V vs. SCE. Representative oxidoreductases, e.g. fungal peroxidase from Coprinus cinereus (rCiP), catalyze the oxidation of AHAs with apparent bimolecular constants (kox) of 7.1·103 to 1.5·107M−1s−1 at pH = 8.5 and 25 °C. The limitingN-芳基异羟肟酸(AHA)是用于氧化还原酶催化的有前途的新型 N-OH 介质。它们是电化学活性化合物,相对于 SCE,氧化还原电位为 0.31-0.41 V。代表性氧化还原酶,例如来自灰鬼伞 (rCiP) 的真菌过氧化物酶,在 pH = 8.5 和 25 °C 时催化表观双分子常数 (kox) 为 7.1·103 至 1.5·107M-1s-1 的 AHA 氧化。底物氧化的限制步骤是化合物 II (Cpd II) 的还原。N-羟基乙酰苯胺 (1a) 和 N-羟基-N-苯基苯甲酰胺 (2a) 的氧化常数通过停流和稳态方法确定,相似。Cpd II 还原率降低的还原率降低发生在 1a 的 pKa = 8.5 和 2a 的 7.7 处。1a 的硝酰基自由基是氧化反应的中间体,在碱性 pH 值下稳定性下降。这些 AHA 的构效关系 (SAR) 在 Marcus 交叉关系的框架内进行分析,并使用从头算
-
An Exceptionally Stable Ti Superoxide Radical Ion: A Novel Heterogeneous Catalyst for the Direct Conversion of Aromatic Primary Amines to Nitro Compounds作者:Gajanan K. Dewkar、Milind D. Nikalje、Iliyas Sayyed Ali、Abhimanyu S. Paraskar、H. S. Jagtap、A. SudalaiDOI:10.1002/1521-3773(20010119)40:2<405::aid-anie405>3.0.co;2-6日期:2001.1.19A matrix-bound superoxide radical anion, generated by treating Ti(OR)4 (R=iPr, nBu) with H2 O2 , is a selective heterogeneous catalyst for the oxidation of anilines to the corresponding nitroarenes with 50 % aqueous H2 O2 [Eq. (1)]. Yields of 82-98 % are obtained, even with anilines bearing electron-withdrawing substituents (R=NO2 , COOH).
表征谱图
-
氢谱1HNMR
-
质谱MS
-
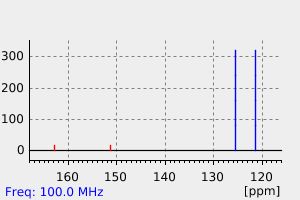
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息