disodium;oxalate
中文名称
——
中文别名
——
英文名称
disodium;oxalate
英文别名
——
CAS
——
化学式
C2Na2O4
mdl
——
分子量
134.0
InChiKey
ZNCPFRVNHGOPAG-UHFFFAOYSA-L
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-9.51
-
重原子数:8
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:80.3
-
氢给体数:0
-
氢受体数:4
反应信息
-
作为反应物:描述:disodium;oxalate 生成 oxalate;1-piperidin-1-ylpropan-2-amine;platinum(2+)参考文献:名称:TAKAMATSU, MASANORI;IKEHDA, JOSIAKI;MATSUI, MUNEHTAKA;NOSEH, NAOSI摘要:DOI:
-
作为产物:描述:参考文献:名称:ZIEGENBALD, SIEGFRIED;SOLYMAR, KAROLY;LOWE, DIETER;UTER, HORST, NEUE HUTTE, 34,(1989) N, C. 43-47摘要:DOI:
-
作为试剂:描述:参考文献:名称:奥沙利铂的制备方法摘要:本发明公开了一种奥沙利铂的制备方法,其包括以下步骤:步骤一,四氯亚铂酸钾与碘化钾、(1R,2R)-环己二胺反应制得碘氨铂;步骤二,步骤一的碘氨铂与硝酸亚铜反应得到胺铂的硝酸盐中间体;步骤三,步骤二的硝酸盐中间体与草酸钠反应制得奥沙利铂。本发明可节约生产成本,降低患者的医疗费用、减少副作用,收率较高。公开号:CN104447877A
文献信息
-
5,6-Benzo analogues of prostaglandin申请人:Miles Laboratories, Inc.公开号:US04238623A1公开(公告)日:1980-12-09Disclosed are prostaglandin analogues having the structural formula, ##STR1## in which: T is selected from the group consisting of carboxyl, alkoxycarbonyl or cyano; M is selected from the group consisting of carbonyl, R-hydroxymethylene or S-hydroxymethylene; L is selected from the group consisting of methylene or methine, provided L is methine only if J is methine; J is selected from the group consisting of methylene, ethylene, R-hydroxymethylene, S-hydroxymethylene or methine, provided J is methine only if L is methine; W is selected from the group consisting of ##STR2## T.sub.1 and T.sub.2 are attached to adjacent carbon atoms; T.sub.1 is selected from the group consisting of hydrogen or phenyl, provided T.sub.1 is phenyl only if T.sub.2 is lower alkyl; T.sub.2 is selected from the group consisting of n-pentyl or lower alkyl, provided T.sub.2 is lower alkyl only if T.sub.1 is phenyl; or T.sub.1 and T.sub.2 are joined together to form an alkylene group of 4 or 6 carbon atoms. Also disclosed are methods for preparing such prostaglandin analogues.本发明涉及具有结构式##STR1##的前列腺素类似物,其中:T选自羧基,烷氧羰基或氰基的群;M选自羰基,R-羟甲基或S-羟甲基的群;L选自亚甲基或亚胺基的群,仅当J为亚胺基时,L才为亚胺基;J选自亚甲基,乙烯基,R-羟甲基,S-羟甲基或亚胺基的群,仅当L为亚胺基时,J才为亚胺基;W选自##STR2##的群;T.sub.1和T.sub.2连接到相邻的碳原子上;T.sub.1选自氢或苯基的群,仅当T.sub.2为较低烷基时,T.sub.1才为苯基;T.sub.2选自正戊基或较低烷基的群,仅当T.sub.1为苯基时,T.sub.2才为较低烷基;或T.sub.1和T.sub.2连接在一起形成4或6个碳原子的烷基。还公开了制备这种前列腺素类似物的方法。
-
Cyclohexanone-5,6-benzo analogues of prostaglandin申请人:Miles Laboratories, Inc.公开号:US04100352A1公开(公告)日:1978-07-11Disclosed are prostaglandin analogues having the structural formula, ##STR1## in which: T is selected from the group consisting of carboxyl, alkoxycarbonyl or cyano; M is selected from the group consisting of carbonyl, R-hydroxymethylene or S-hydroxymethylene; L is selected from the group consisting of methylene or methine, provided L is methine only if J is methine; J is selected from the group consisting of methylene, ethylene, R-hydroxymethylene, S-hydroxymethylene or methine, provided J is methine only if L is methine; W is selected from the group consisting of ##STR2## T.sub.1 and T.sub.2 are attached to adjacent carbon atoms; T.sub.1 is selected from the group consisting of hydrogen or phenyl, provided T.sub.1 is phenyl only if T.sub.2 is lower alkyl; T.sub.2 is selected from the group consisting of n-pentyl or lower alkyl, provided T.sub.2 is lower alkyl only if T.sub.1 is phenyl; Or T.sub.1 and T.sub.2 are joined together to form an alkylene group of 4 or 6 carbon atoms. Also disclosed are methods for preparing such prostaglandin analogues.本发明涉及结构式为##STR1##的前列腺素类似物,其中:T选自羧基,烷氧羰基或氰基的群组中;M选自羰基,R-羟甲基或S-羟甲基的群组中;L选自亚甲基或亚胺基的群组中,仅当J为亚胺基时,L才为亚胺基;J选自亚甲基,乙烯基,R-羟甲基,S-羟甲基或亚胺基的群组中,仅当L为亚胺基时,J才为亚胺基;W选自##STR2##的群组中;T.sub.1和T.sub.2附着在相邻的碳原子上;T.sub.1选自氢或苯基的群组中,仅当T.sub.2为较低的烷基时,T.sub.1才为苯基;T.sub.2选自正戊基或较低的烷基的群组中,仅当T.sub.1为苯基时,T.sub.2才为较低的烷基;或T.sub.1和T.sub.2结合形成4或6个碳原子的烷基。本发明还涉及制备这种前列腺素类似物的方法。
-
5,6-Benza analogues of prostaglandin F申请人:Miles Laboratories, Inc.公开号:US04100353A1公开(公告)日:1978-07-11Disclosed are prostaglandin analogues having the structural formula, ##STR1## in which: T is selected from the group consisting of carboxyl, alkoxycarbonyl or cyano; M is selected from the group consisting of carbonyl, R-hydroxymethylene or S-hydroxymethylene; L is selected from the group consisting of methylene or methine, provided L is methine only if J is methine; J is selected from the group consisting of methylene, ethylene, R-hydroxymethylene, S-hydroxymethylene or methine, provided J is methine only if L is methine; W is selected from the group consisting of ##STR2## T.sub.1 and T.sub.2 are attached to adjacent carbon atoms; T.sub.1 is selected from the group consisting of hydrogen or phenyl, provided T.sub.1 is phenyl only if T.sub.2 is lower alkyl; T.sub.2 is selected from the group consisting of n-pentyl or lower alkyl, provided T.sub.2 is lower alkyl only if T.sub.1 is phenyl; or T.sub.1 and T.sub.2 are joined together to form an alkylene group of 4 to 6 carbon atoms. Also disclosed are methods for preparing such prostaglandin analogues.
-
5,6-Benzo analogues or prostaglandin E申请人:Miles Laboratories, Inc.公开号:US04096336A1公开(公告)日:1978-06-20Disclosed are prostaglandin analogues having the structural formula, ##STR1## in which: T is selected from the group consisting of carboxyl, alkoxycarbonyl or cyano; M is selected from the group consisting of carbonyl, R-hydroxymethylene or S-hydroxymethylene; L is selected from the group consisting of methylene or methine, provided L is methine only if J is methine; J is selected from the group consisting of methylene, ethylene, R-hydroxymethylene, S-hydroxymethylene or methine, provided J is methine only if L is methine; W is selected from the group consisting of --CH.sub.2 --CH-- or trans --CH.dbd.C--; T.sub.1 and T.sub.2 are attached to adjacent carbon atoms; T.sub.1 is selected from the group consisting of hydrogen or phenyl, provided T.sub.1 is phenyl only if T.sub.2 is lower alkyl; T.sub.2 is selected from the group consisting of n-pentyl or lower alkyl, provided T.sub.2 is lower alkyl only if T.sub.1 is phenyl; Or T.sub.1 and T.sub.2 are joined together to form an alkylene group of 4 or 6 carbon atoms. Also disclosed are methods for preparing such prostaglandin analogues.公开了具有结构式##STR1##的前列腺素类似物,其中:T选自羧基,烷氧基甲酰基或氰基的群;M选自羰基,R-羟甲基亚甲基或S-羟甲基亚甲基的群;L选自甲基或亚甲基,仅当J为亚甲基时,L才为亚甲基;J选自甲基,乙烯基,R-羟甲基亚甲基,S-羟甲基亚甲基或亚甲基的群,仅当L为亚甲基时,J才为亚甲基;W选自--CH.sub.2 --CH--或trans--CH.dbd.C--的群;T.sub.1和T.sub.2附着在相邻的碳原子上;T.sub.1选自氢或苯基,仅当T.sub.2为较低的烷基时,T.sub.1才为苯基;T.sub.2选自n-戊基或较低的烷基,仅当T.sub.1为苯基时,T.sub.2才为较低的烷基;或T.sub.1和T.sub.2结合形成4或6个碳原子的烷基。还公开了制备这种前列腺素类似物的方法。
-
5,6-Benzo analogues of prostaglandin E申请人:Miles Laboratories, Inc.公开号:US04097516A1公开(公告)日:1978-06-27Disclosed are prostaglandin analogues having the structural formula, ##STR1## in which: T is selected from the group consisting of carboxyl, alkoxycarbonyl or cyano; M is selected from the group consisting of carbonyl, R-hydroxymethylene or S-hydroxymethylene; L is selected from the group consisting of methylene or methine, provided L is methine only if J is methine; J is selected from the group consisting of methylene, ethylene, R-hydroxymethylene, S-hydroxymethylene or methine, provided J is methine only if L is methine; W is selected from the group consisting of --CH.sub.2 --CH-- or trans --CH.dbd.C--; T.sub.1 and T.sub.2 are attached to adjacent carbon atoms; T.sub.1 is selected from the group consisting of hydrogen or phenyl, provided T.sub.1 is phenyl only if T.sub.2 is lower alkyl; T.sub.2 is selected from the group consisting of n-pentyl or lower alkyl, provided T.sub.2 is lower alkyl only if T.sub.1 is phenyl; Or T.sub.1 and T.sub.2 are joined together to form an alkylene group of 4 or 6 carbon atoms. Also disclosed are methods for preparing such prostaglandin analogues.本发明涉及具有结构式##STR1##的前列腺素类似物,其中:T选自羧基,烷氧基甲酰基或氰基的群体;M选自羰基,R-羟甲基亚甲基或S-羟甲基亚甲基的群体;L选自亚甲基或亚胺基,仅当J为亚胺基时,L才为亚胺基;J选自亚甲基,乙烯基,R-羟甲基亚甲基,S-羟甲基亚甲基或亚胺基的群体,仅当L为亚胺基时,J才为亚胺基;W选自--CH.sub.2 --CH--或顺式--CH.dbd.C--的群体;T.sub.1和T.sub.2连接到相邻的碳原子上;T.sub.1选自氢或苯基,仅当T.sub.2为低碳基时,T.sub.1才为苯基;T.sub.2选自正戊基或低碳基,仅当T.sub.1为苯基时,T.sub.2才为低碳基;或T.sub.1和T.sub.2结合形成4或6个碳原子的烷基。还公开了制备这种前列腺素类似物的方法。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
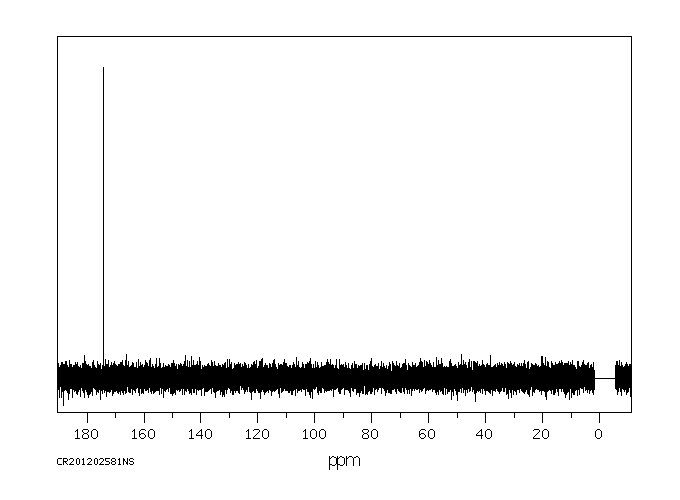
碳谱13CNMR
-
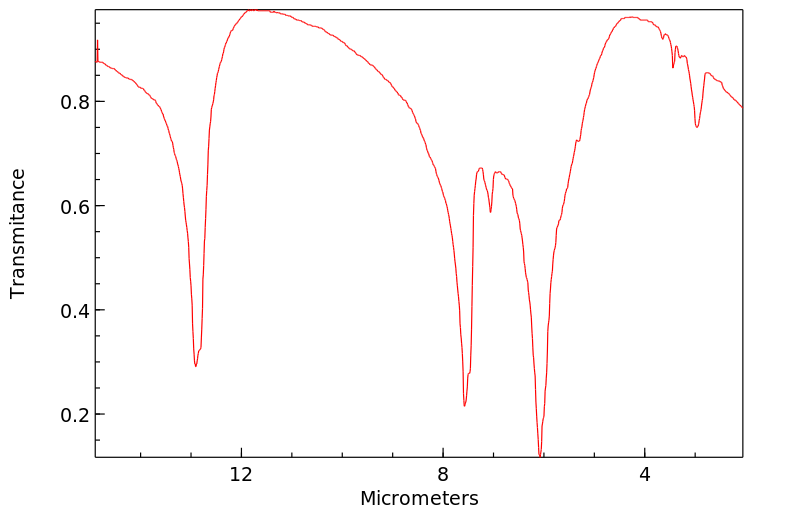
红外IR
-
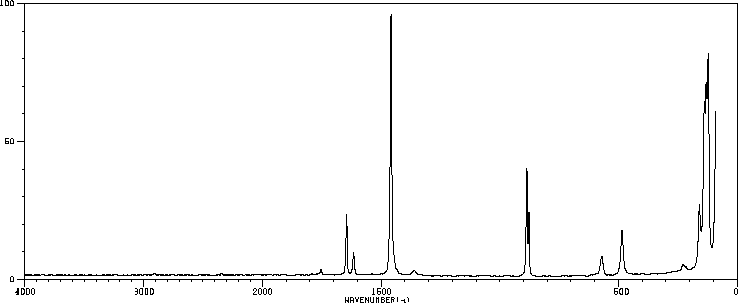
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸