丁酸苯酯 | 4346-18-3
中文名称
丁酸苯酯
中文别名
正丁酸苯酯
英文名称
butanoic acid phenyl ester
英文别名
butyric acid phenyl ester;phenyl butanoate;phenyl butyrate
CAS
4346-18-3
化学式
C10H12O2
mdl
MFCD00083050
分子量
164.204
InChiKey
IGVPBCZDHMIOJH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:225°C (estimate)
-
密度:1.0382
-
LogP:2.62
-
物理描述:Colourless liquid; Sweet floral aroma
-
溶解度:Practically insoluble or insoluble in water; soluble in ether
-
折光率:1.448-1.494
-
保留指数:1245
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:12
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
制备方法与用途
用途:用作溶剂,并且也应用于香料工业。
上下游信息
反应信息
-
作为反应物:参考文献:名称:WO2007/44796摘要:公开号:
-
作为产物:描述:butyric acid silicic acid-anhydride 在 C6H5OH 作用下, 生成 丁酸苯酯参考文献:名称:Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Si: MVol.C, 125, page 348 - 350摘要:DOI:
文献信息
-
<sup>13</sup>C-NMR. Spectral Differences between Corresponding Methyl Esters, Phenyl Esters and 2-Substituted Chromones作者:Urs SéquinDOI:10.1002/hlca.19810640824日期:1981.12.16The 13C-NMR. spectra of 2-substituted chromones (3) are compared with the data of the analogous methyl and phenyl esters (1 and 2). The chemical shift differences found are most prominent for the C-atoms in β-position to the ester carbonyl and chromone C(2), respectively. These shift differences are discussed in terms of conformational differences between the esters 1 and 2 and the analogous chromones
-
Photosensitive compound申请人:Wako Pure Chemical Industries, Ltd.公开号:US05250669A1公开(公告)日:1993-10-05Photosensitive compounds having preferably a functional group such as --SO.sub.2 Cl, --SO.sub.3 H, --SO.sub.3 R, ##STR1## (R, R', R" being alkyl) on a terminal benzene or naphthalene ring connected via a methylene group and ##STR2## moiety are improved in sensitivity to light and thermal stability, and thus useful in a photo resist.具有偏好功能基团的光敏化合物,例如--SO.sub.2 Cl,--SO.sub.3 H,--SO.sub.3 R,##STR1##(R,R',R"为烷基)连接通过亚甲基基团的末端苯或萘环和##STR2##基团,在光敏度和热稳定性方面得到改善,因此在光刻胶中有用。
-
[EN] AFFINITY-ASSISTED PROTEIN MODIFICATION AND RECYCLING<br/>[FR] MODIFICATION ET RECYCLAGE DE PROTÉINES BASÉS SUR LEUR AFFINITÉ申请人:UNIV CALIFORNIA公开号:WO2016118770A1公开(公告)日:2016-07-28The present invention provides methods for preparing a protein conjugate having a defined number of conjugate groups. The method includes: forming a mixture containing a macrocyclic matrix material and a plurality of proteins; eluting the proteins from the macrocyclic matrix material to obtain a first separated protein fraction and a second separated protein fraction, wherein substantially all of the proteins in the first separated protein fraction have the same number of handle moieties; contacting the handle moieties with a conversion reagent under conditions sufficient to convert the handle moieties in the first separated protein fraction to reactive moieties; and contacting the reactive moieties with a conjugation reagent under conditions sufficient to form a plurality of protein conjugates, wherein substantially all of the protein conjugates in the plurality have the same number of conjugate groups. Also disclosed are methods for recovering enzymes and other proteins from mixtures for isolation and/or reuse of the enzymes and proteins.本发明提供了制备具有定义数量结合基团的蛋白共轭物的方法。该方法包括:形成含有大环矩阵材料和多种蛋白质的混合物;从大环矩阵材料中洗脱蛋白质,以获得第一分离的蛋白质分数和第二分离的蛋白质分数,其中第一分离的蛋白质分数中几乎所有蛋白质具有相同数量的手柄基团;将手柄基团与转化试剂接触,在足以将第一分离的蛋白质分数中的手柄基团转化为反应性基团的条件下;将反应性基团与结合试剂接触,在足以形成多种蛋白共轭物的条件下,其中多数蛋白共轭物具有相同数量的结合基团。还公开了从混合物中回收酶和其他蛋白质以便分离和/或重复使用酶和蛋白质的方法。
-
Cobalt‐Catalyzed Radical Hydroamination of Alkenes with <i>N</i> ‐Fluorobenzenesulfonimides作者:Tao Qin、Guowei Lv、Qi Meng、Ge Zhang、Tao Xiong、Qian ZhangDOI:10.1002/anie.202110178日期:2021.12N-fluorobenzenesulfonimide (NFSI) and its analogues as both nitrogen source and oxidant was successfully disclosed. A variety of alkenes, including aliphatic alkenes, styrenes, α, β-unsaturated esters, amides, acids, as well as enones, were all compatible to provide desired amination products. Mechanistic experiments suggest that the reaction underwent a metal-hydride-mediated hydrogen atom transfer (HAT) with alkene
-
Conjugate reduction of α,β-unsaturated esters and amides with tributyltin hydride in the presence of magnesium bromide diethyl etherate作者:Satomi Hirasawa、Yoshie Tajima、Yoko Kameda、Hajime NaganoDOI:10.1016/j.tet.2007.08.059日期:2007.11We report herein that the conjugate reduction of α,β-unsaturated esters and amides, such as aryl acrylates, pantolactone esters of acrylic acids, diethyl itaconate and N-crotonylcamphorsultam, with tributyltin hydride proceeded in moderate to high yields in the presence of magnesium bromide diethyl etherate. The effect of metal halide enhancing the yields is also described.
表征谱图
-
氢谱1HNMR
-
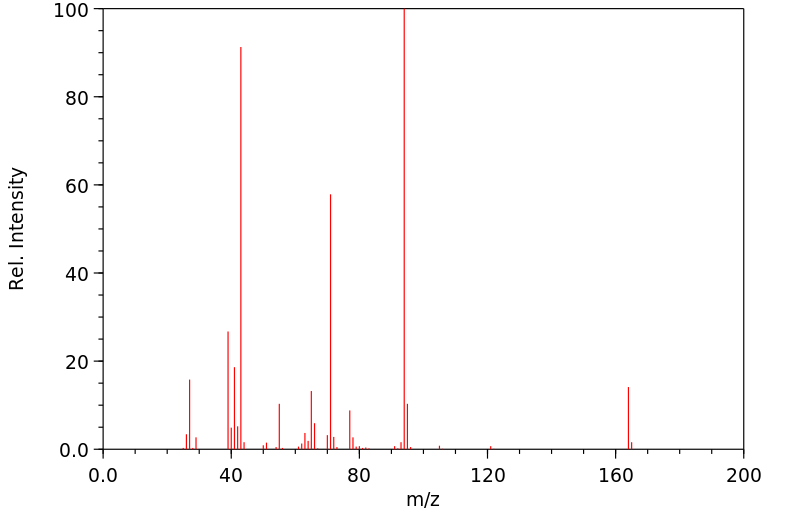
质谱MS
-
碳谱13CNMR
-
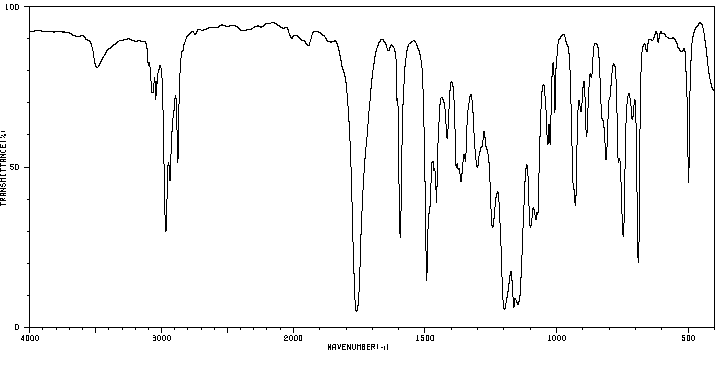
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰亚胺四聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺六聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺-酰胺-PEG8-四氟苯酚酯
马来酰亚胺-四聚乙二醇-五氟苯酯
马来酰亚胺-三聚乙二醇-五氟苯酚酯
靛酚乙酸酯
阿立哌唑标准品002
间硝基苯基戊酸酯
间氯苯乙酸乙酯
间乙酰苯甲酸
钾4-乙酰氧基苯磺酸酯
酚醛乙酸酯
邻苯二酚二乙酸酯
邻甲苯基环己甲酸酯
邻甲氧基苯乙酸酯
辛酸苯酯
辛酸对甲苯酚酯
辛酸五氯苯基酯
辛酸-(3-氯-苯基酯)
辛酰溴苯腈
苯酰胺,3,4-二(乙酰氧基)-N-[6-氨基-1,2,3,4-四氢-1-(4-甲氧苯基)-3-甲基-2,4-二羰基-5-嘧啶基]-
苯酚-乳酸
苯酚,4-异氰基-,乙酸酯(ester)
苯酚,4-[(四氢-2H-吡喃-2-基)氧代]-,乙酸酯
苯酚,3-(1,1-二甲基乙基)-,乙酸酯
苯酚,2-溴-3-(二溴甲基)-5-甲氧基-,乙酸酯
苯甲醇,4-(乙酰氧基)-3,5-二甲氧基-
苯甲酸,4-(乙酰氧基)-2-氟-
苯氧基氯乙酸苯酯
苯基金刚烷-1-羧酸酯
苯基氰基甲酸酯
苯基庚酸酯
苯基庚-6-炔酸酯
苯基己酸酯
苯基呋喃-2-羧酸酯
苯基吡啶-2-羧酸酯
苯基十一碳-10-烯酸酯
苯基乙醛酸酯
苯基乙酸酯-d5
苯基丙二酸单苯酯
苯基丙-2-炔酸酯
苯基丁-2,3-二烯酸酯
苯基4-乙基环己烷羧酸
苯基3-乙氧基-3-亚氨基丙酸盐
苯基2-(苯磺酰基)乙酸酯
苯基2-(4-甲氧基苯基)乙酸酯
苯基2-(2-甲氧基苯基)乙酸酯
苯基2-(2-甲基苯基)乙酸酯
苯基-乙酸-(2-甲酰基-苯基酯)
苯基-乙酸-(2-环己基-苯基酯)