三乙二醇二异辛酸酯 | 94-28-0
中文名称
三乙二醇二异辛酸酯
中文别名
三(乙二醇)双(2-乙基己酸酯);三甘醇二异辛酸酯;三甘醇二-2-乙基己酸酯;三乙二醇双(2-乙酸己酯);双(异辛酸)三乙二醇酯
英文名称
triethylene glycol di-2-ethylhexanoate
英文别名
Triethylene glycol bis(2-ethylhexanoate);2-[2-[2-(2-ethylhexanoyloxy)ethoxy]ethoxy]ethyl 2-ethylhexanoate
CAS
94-28-0
化学式
C22H42O6
mdl
——
分子量
402.572
InChiKey
FRQDZJMEHSJOPU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:−50 °C(lit.)
-
沸点:344 °C(lit.)
-
密度:0.97 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
LogP:6.1 at 25℃
-
物理描述:DryPowder; Liquid; OtherSolid; PelletsLargeCrystals
-
稳定性/保质期:
在常温常压下稳定,避免与不相容材料接触。它会与强氧化剂反应。
计算性质
-
辛醇/水分配系数(LogP):5.4
-
重原子数:28
-
可旋转键数:21
-
环数:0.0
-
sp3杂化的碳原子比例:0.91
-
拓扑面积:71.1
-
氢给体数:0
-
氢受体数:6
安全信息
-
TSCA:Yes
-
安全说明:S24/25
-
WGK Germany:-
-
海关编码:2918990090
-
危险品标志:Xn,Xi
-
RTECS号:MO7725000
-
危险类别码:R22,R36/37/38
-
储存条件:密封储存,应置于阴凉干燥处。
SDS
| Name: | Triethylene glycol bis(2-ethylhexanoate) Material Safety Data Sheet |
| Synonym: | |
| CAS: | 94-28-0 |
Synonym:
SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 94-28-0 | Triethylene glycol bis(2-ethylhexanoat | 100 | 202-319-2 |
Risk Phrases: None Listed.
SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW Not available. Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
May cause respiratory tract irritation.
Chronic:
Not available.
SECTION 4 - FIRST AID MEASURES
Eyes:
Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
SECTION 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
SECTION 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
SECTION 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use only in a well-ventilated area. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a cool, dry, well-ventilated area away from incompatible substances.
SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 94-28-0: Personal Protective Equipment
Eyes:
Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 219 deg C @ 5.00mm Hg
Freezing/Melting Point: -50 deg C
Autoignition Temperature: 725 deg F ( 385.00 deg C)
Flash Point: 390 deg F ( 198.89 deg C)
Explosion Limits, lower: 0.46 volume %
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Negligible.
Specific Gravity/Density: .9670g/cm3
Molecular Formula: C22H42O6
Molecular Weight: 402.57
SECTION 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Will not occur.
SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 94-28-0: MO7725000
LD50/LC50:
CAS# 94-28-0: Dermal, guinea pig: LD50 = >20 mL/kg; Oral, mouse: LD50 = >3200 mg/kg; Oral, rat: LD50 = 31 gm/kg; Skin, rabbit: LD50 = 14100 uL/kg.
Carcinogenicity:
Triethylene glycol bis(2-ethylhexanoate) - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
SECTION 12 - ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: Fathead Minnow: > 100 microliters/l; 96 hr.
SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
SECTION 14 - TRANSPORT INFORMATION IATA Not regulated as a hazardous material. IMO Not regulated as a hazardous material. RID/ADR Not regulated as a hazardous material.
SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases: S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 94-28-0: 1 Canada CAS# 94-28-0 is listed on Canada's DSL List. CAS# 94-28-0 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 94-28-0 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
MSDS Creation Date: 6/02/1998 Revision #3 Date: 2/04/2004 The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
应用
目前,三乙二醇二异辛酸酯广泛应用于聚乙烯醇缩丁醛(PVB)安全膜、合成橡胶、聚氯乙烯(PVC)、密封材料等的制备中,具有极大的应用价值。
用途本品是一种溶剂型耐寒增塑剂,具有优良的低温性、耐久性、耐油性、耐紫外线照射和抗静电性,且粘度低并具有一定的润滑性。它可以与多种天然树脂和合成橡胶相容,并可溶于多数有机溶剂,而不溶于矿物油。在增塑糊中具有触变性,特别适合特殊用途的应用。
本品是聚乙烯醇缩丁醛(PVB安全玻璃)和合成橡胶的特效增塑剂,能赋予它们低温性能和低挥发性。也可用于涤布、粘结剂及密封材料,是PVC、PS、乙基纤维素、硝醛纤维素等的增塑剂。在含蓖麻油的聚乙烯醇缩丁醛布基涂料中使用时,能改善严寒条件下的柔顺性。此外,它还可用于丁二烯-丙烯腈类耐油合成橡胶和聚乙烯乳胶漆的配方中,通常用量比邻苯二甲酸二辛酯或磷酸三甲苯酯都要低。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-乙基己酸甲酯 methyl 2-ethylhexanoate 816-19-3 C9H18O2 158.241 2-乙基-己酸稀土盐 2-Ethylhexanoic acid 149-57-5 C8H16O2 144.214
反应信息
-
作为产物:描述:参考文献:名称:[EN] SYNTHESIS OF TRIETHYLENE GLYCOL BIS(2-ETHYLHEXANOATE)
[FR] SYNTHÈSE DE TRIÉTHYLÈNE GLYCOL BIS(2-ÉTHYLHEXANOATE)摘要:提供了一种用三乙二醇对甲基-2-乙基己酸酯进行酯交换反应以产生三乙二醇二-2-乙基己酸酯的方法。在该过程中,将甲基-2-乙基己酸酯与三乙二醇混合形成第一混合物。在催化剂的存在下加热第一混合物,形成包含甲醇和三乙二醇二-2-乙基己酸酯的第二混合物。将甲醇从第二混合物中分离出来,得到三乙二醇二-2-乙基己酸酯。Na2CO3、CS2CO3、K2CO3、Rb2CO3、甲基钠或异丙酸钛是适合的催化剂。公开号:WO2020102128A1
文献信息
-
Process for preparing polyol esters申请人:Adamzik Michael公开号:US20110087045A1公开(公告)日:2011-04-14The present invention relates to a process for preparing polyol esters by reacting polyols with linear or branched aliphatic monocarboxylic acids having 3 to 20 carbon atoms by partial recycling of the aliphatic monocarboxylic acid removed into the esterification reaction or into subsequent esterification batches.
-
PROCESS FOR PREPARING POLYOL ESTERS申请人:Frey Guido D.公开号:US20120190883A1公开(公告)日:2012-07-26The present invention relates to a process for preparing polyol esters by reacting polyols with linear or branched aliphatic monocarbocxylic acids having 3 to 20 carbon atoms, the reaction taking place in the presence of a Lewis acid comprising at least one element from groups 4 to 14 of the Periodic Table of the Elements as catalyst, and in the presence of an adsorbent, the reaction product being subjected subsequently to a steam treatment.
-
[EN] METHOD OF PREPARING GLYCOL ESTERS WITH LOW COLOR AND PEROXIDE CONTENT<br/>[FR] PROCÉDÉ DE PRÉPARATION D'ESTERS DE GLYCOL À FAIBLE TENEUR EN COULEUR ET EN PEROXYDE申请人:SIBUR HOLDING PUBLIC JOINT STOCK CO公开号:WO2019059800A1公开(公告)日:2019-03-28The present invention relates to the field of preparing glycol esters used as plasticizers in the manufacture of films for multilayered glass. In particular, the invention relates to a method for preparing triethylene glycol ester of 2-ethylhexanoic acid. The claimed method includes the steps of esterification, clarification of a crude ester by treating thereof with hydrogen peroxide and alkali metal metasilicate at a temperature of from 70 to 200ºC, inclusive, and allowing to stand for 0.5 to 1.5 hours, inclusive, to obtain a clarified ester, neutralization of acidic compounds in the clarified ester by treating thereof with an alkaline solution, filtration, and drying of the ester. The technical result of the invention is the development of an economical and safe method of preparing glycol esters that are characterized by low values of color, acidity and peroxide content.
-
[EN] PHOTOCHROMIC AND ELECTROCHROMIC DIARYLETHENE COMPOUNDS WITH IMPROVED PHOTOSTABILITY AND SOLUBILITY<br/>[FR] COMPOSÉS DIARYLÉTHÈNE PHOTOCHROMIQUES ET ÉLECTROCHROMES PRÉSENTANT UNE PHOTOSTABILITÉ ET UNE SOLUBILITÉ AMÉLIORÉES申请人:SWITCH MAT INC公开号:WO2020198868A1公开(公告)日:2020-10-08A diarylethene compound reversibly convertible under photochromic and electrochromic conditions between a ring-open isomer of Formula (1A) and a ring-closed isomer of Formula (IB) wherein R5 is a substituted phenyl ring and Re is a substituted thiophene ring is provided. The photochromic-electrochromic diarylethene compound of Formula (1A)/(1B) have improved photochromic, electrochromic or photochromic and electrochromic properties, and is useful to provide variation of the light transmission properties of optical filters. The compound also possesses improved solubility making it suitable for incorporation in commercial products..
-
Method for Producing Polyol Esters申请人:OXEA GmbH公开号:US20150344400A1公开(公告)日:2015-12-03A process for preparing polyol esters by reacting polyols with linear or branched aliphatic monocarboxylic acids having 3 to 20 carbon atoms, is characterized in that a mixture of the starting compounds is allowed to react in the presence of a Lewis acid containing at least one element of groups 4 to 14 of the periodic table of the elements as a catalyst and in the presence of an adsorbent with removal of the water formed, and then the crude ester obtained is aftertreated by adding a further adsorbent which is an acidic activated carbon having a pH of 1 to 6.5.
表征谱图
-
氢谱1HNMR
-
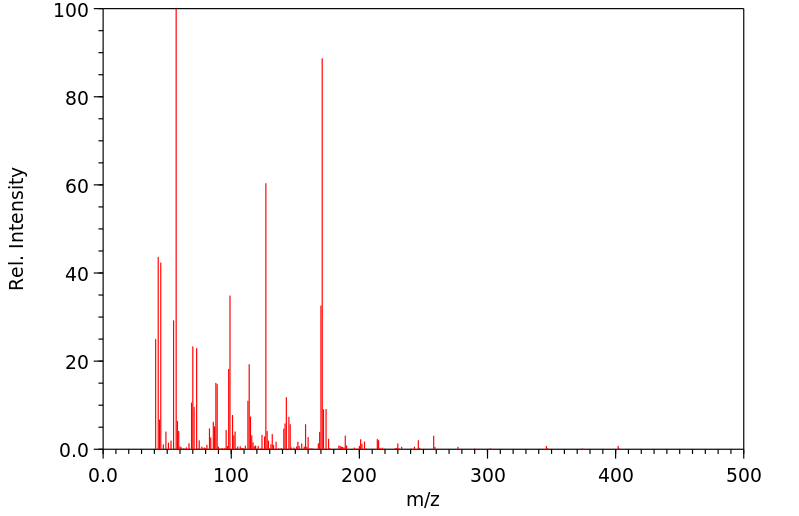
质谱MS
-
碳谱13CNMR
-
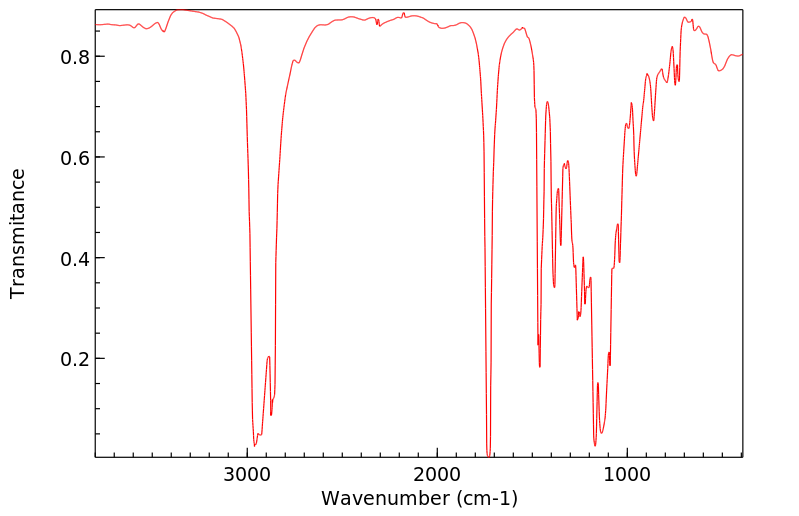
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯