二己硫 | 10496-15-8
中文名称
二己硫
中文别名
二己基二硫醚;二己二硫化物
英文名称
dihexyl disulfide
英文别名
di-n-hexyl disulfide;1,2-dihexyldisulfane;n-hexyl disulfide;1-(hexyldisulfanyl)hexane;di-n-hexyldisulphide
CAS
10496-15-8
化学式
C12H26S2
mdl
MFCD00027300
分子量
234.47
InChiKey
GJPDBURPGLWRPW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-47.99°C
-
沸点:229-230°C
-
密度:0,92 g/cm3
-
闪点:>110°(230°F)
-
LogP:7.245 (est)
-
保留指数:1927
-
稳定性/保质期:
遵照规格使用和储存则不会分解。
计算性质
-
辛醇/水分配系数(LogP):5.6
-
重原子数:14
-
可旋转键数:11
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:50.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
海关编码:2930909090
-
安全说明:S23,S24/25
-
包装等级:III
-
危险类别:9
-
危险性防范说明:P261,P264,P271,P273,P302+P352,P305+P351+P338
-
危险品运输编号:3334
-
危险性描述:H315,H319,H335,H411,H372
-
储存条件:密封于阴凉干燥处。
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Oxyhalogenation of thiols and disulfides into sulfonyl chlorides/bromides using oxone-KX (X = Cl or Br) in water摘要:一种简单高效的方法,通过使用水作为溶剂,利用氧杂合物-KX(X = Cl或Br)对硫醇和二硫化物进行氧卤化反应,合成磺酰氯/溴。DOI:10.1039/c4gc00246f
-
作为产物:参考文献:名称:Synthesis of Disulfides Containing a Corrinoid Head Group for Preparation of Self-Assembled Monolayers摘要:描述了三种新型烷烃二硫化物的合成,这些二硫化物的头基源自维生素B12,分别为14a、14b和15。采用对称二硫化物15,具有两个羟基钴质头基,制备了金表面的自组装单层。通过时间飞行二次离子质谱(TOF-SIMS)和电子能谱(ESCA)对金表面的涂层进行了分析。DOI:10.1055/s-2005-871941
-
作为试剂:参考文献:名称:Rate constants and equilibrium constants for thiol-disulfide interchange reactions involving oxidized glutathione摘要:DOI:10.1021/ja00526a042
文献信息
-
Hydrotalcite Clay-catalysed Air Oxidation of Thiols作者:Masao Hirano、Hiroyuki Monobe、Sigetaka Yakabe、Takashi MorimotoDOI:10.1039/a808922a日期:——Hydrotalcite is an efficient catalyst for air oxidation of a variety of aromatic, aliphatic and alicyclic thiols in hexane, affording the corresponding disulfides in excellent to quantitative yields under mild and neutral conditions.
-
An Organodiselenide with Dual Mimic Function of Sulfhydryl Oxidases and Glutathione Peroxidases: Aerial Oxidation of Organothiols to Organodisulfides作者:Vandana Rathore、Aditya Upadhyay、Sangit KumarDOI:10.1021/acs.orglett.8b02756日期:2018.10.5peroxidase (GPx) enzymes for oxidation of thiols by oxygen and hydrogen peroxide, respectively, into disulfides has been presented. The developed catalyst oxidizes an array of organothiols into respective disulfides in practical yields by using aerial O2 to avoid any reagents/additives, base, and light source. The synthesized diselenide also catalyzes the reduction of hydrogen peroxide into water by following
-
Simple Method for the Preparation of Symmetrical Alkyl and Aryl Disulfides with Alkyl Sulfonyl Halides in Nitrogenous Base作者:Firdous Imran Ali、Imran Ali Hashmi、Bina S. Siddiqui、Munawwer RasheedDOI:10.1080/00397910701459498日期:2007.8Abstract In the present study, the reaction of thiols with alkyl sulfonyl halides was carried out in a nitrogenous base to compare the reactivity of ‐SH with that of ‐OH, which, however, led to the formation of disulfides. The reaction achieved as a result offers the use of an inexpensive reagent, quantitative yields of the product, and simplicity for the formation of the S‐S bond.
-
Alumina: an Efficient and Reusable Catalyst for the Oxidative Coupling of Thiols with DMSO作者:Masao Hirano、Sigetaka Yakabe、Hiroyuki Monobe、Takashi MorimotoDOI:10.1039/a802772b日期:——An inexpensive combination of common laboratory reagents, dimethyl sulfoxide (DMSO) and chromatographic neutral alumina, gives an efficient, selective, and high-yielding oxidation of aromatic, aliphatic and alicyclic thiols to the corresponding disulfides in excellent yields under relatively mild conditions.
-
Activation and synthetic applications of thiostannanes. Efficient conversion of thiols into disulfides
表征谱图
-
氢谱1HNMR
-
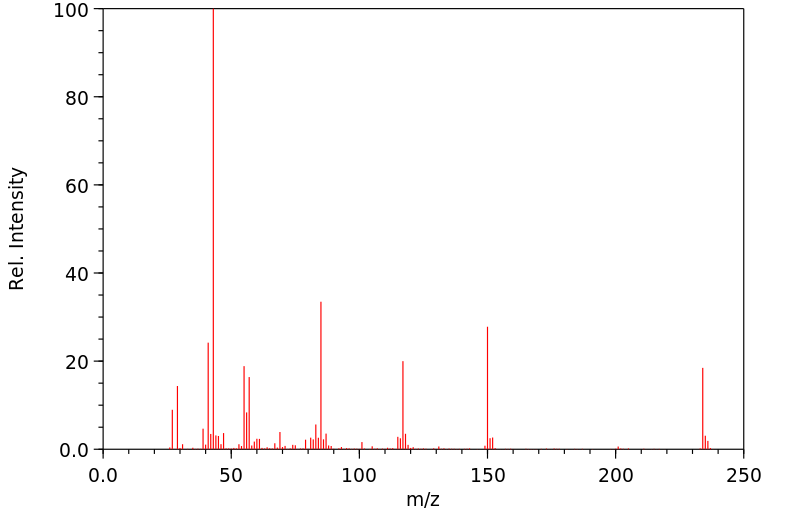
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高胱胺
胱胺
福多司坦杂质
甲基异戊基二硫醚
甲基异丙基二硫醚
甲基半胱胺
甲基丙基二硫醚
甲基丙-1-烯基二硫醚
甲基[2-甲基-1-(甲硫基)丁基]过硫化物
甲基3-甲基-1-丁烯基二硫醚
甲基-D6 二硫醚
氧化福美双
次氮基-氰基二硫基-甲烷
敌灭生
戊基甲基二硫醚
异丙基二硫醚
哌啶并,3-[2-(2-乙基苯基)肼基]-
叔丁基硫基二甲基氨基二硫代甲酸酯
叔丁基二硫
反式丙烯基丙基二硫
双羟甲基二硫化物
双正癸基二硫醚
双十六烷基二硫化物
双(十二烷基硫烷基硫代羰基)二硫化物
双(十三氟己基)二硫醚
双(三氟硫代乙酰基)二硫化物
双(2,2-二乙氧基乙基)二硫化物
双(2,2,2-三氟乙基)二硫化物
双(16-羟基十六烷基)二硫化物
双(11-羟基十一烷基)二硫化物
双(1,2-二甲基-2-氯丙基)二硫化物
原文:多(2,3-环硫烷基)二硫化物,但查不到猜测:双(2,3-环硫丙基)二硫化物
二黄原酸
二肉豆蔻基二硫醚
二硫氨磷汀
二硫化二正丁基黄原酸酯
二硫化二异丙基黄原酸酯
二硫化,二环辛基
二硫化,二(1-羰基十六烷基)
二硫代氨基甲酰二硫醚
二硫代戊酯
二甲基二硫
二甲基-13C2二硫
二环己基二硫化物
二环丙基二硫
二氯-[(甲基二硫烷基)甲氧基]甲烷
二氯-(甲基二硫烷基)甲烷
二正辛基二硫
二正庚基二硫醚
二正壬基二硫醚