二正庚基二硫醚 | 10496-16-9
中文名称
二正庚基二硫醚
中文别名
二庚基二硫醚
英文名称
diheptyldisulfide
英文别名
di-n-heptyl disulfide;Diheptyl disulfide;1-(heptyldisulfanyl)heptane
CAS
10496-16-9
化学式
C14H30S2
mdl
MFCD00039981
分子量
262.524
InChiKey
IFGAFLQUAVLERP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-37.99°C
-
沸点:147 °C / 5mmHg
-
密度:0.91
-
稳定性/保质期:
遵照规格使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):6.7
-
重原子数:16
-
可旋转键数:13
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:50.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2930909090
-
储存条件:密封于阴凉干燥处。
SDS
二庚基二硫醚 修改号码:5
模块 1. 化学品
产品名称: Diheptyl Disulfide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 二庚基二硫醚
百分比: >94.0%(GC)
CAS编码: 10496-16-9
俗名: Heptyl Disulfide
分子式: C14H30S2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
二庚基二硫醚 修改号码:5
模块 5. 消防措施
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 147 °C/0.7kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.91
溶解度:
[水] 无资料
[其他溶剂]
二庚基二硫醚 修改号码:5
模块 9. 理化特性
溶于: 甲醇
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
二庚基二硫醚 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Diheptyl Disulfide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 二庚基二硫醚
百分比: >94.0%(GC)
CAS编码: 10496-16-9
俗名: Heptyl Disulfide
分子式: C14H30S2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
二庚基二硫醚 修改号码:5
模块 5. 消防措施
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 147 °C/0.7kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.91
溶解度:
[水] 无资料
[其他溶剂]
二庚基二硫醚 修改号码:5
模块 9. 理化特性
溶于: 甲醇
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
二庚基二硫醚 修改号码:5
模块16 - 其他信息
N/A
反应信息
-
作为反应物:参考文献:名称:Sano, Shigeki; Tanba, Michiko; Nagao, Yoshimitsu, Heterocycles, 1994, vol. 38, # 3, p. 481 - 486摘要:DOI:
-
作为产物:参考文献:名称:TiCl4/Sm体系还原硫氰酸烷基酯制备二烷基二硫化物摘要:摘要 通过在 0°C 的 THF 中用 TiCl4/Sm 系统还原烷基硫氰酸酯,可以很容易地以中等至良好的收率制备二烷基二硫化物。DOI:10.1080/00397919708004141
文献信息
-
Silica-supported ICl as a novel heterogeneous system for the rapid and selective oxidation of thiols to symmetrical disulfides作者:Bahador Karami、Mahnaz Farahi、Morteza Montazerozohori、Masoud Nasr-EsfahaniDOI:10.1080/17415993.2011.606625日期:2011.10.1application as a volumetric reagent (8). Iodine monochloride (1) has also been used as an iodinating agent without involving a catalyst. It has two crystalline forms α and β that contain non-planar chains of ICl (1) molecules arranged in a zig-zag manner (9). This compound (1) has high vapor pressure and, similar to I2 molecules, evaporates easily. While it has been used frequently, there is some hazard while近年来,一氯化碘(1)被广泛用于各种有机转化,如活化芳香底物的碘化(1)、6-碘喹唑啉-4(3H)-酮衍生物的合成(2)、核酸的碘化在有机溶剂 (3) 中,与 CF3COOAg、CH3COONa 或 (CH3COO)2Pb 反应生成亲电子碘 (4),使用 ICl-NaOMe 或 ICl-吡啶对受阻烯基硼酸酯进行碘脱硼和氯脱硼的立体选择性( 5),除了支链 E-烯烃 (6),从相应的 β-酮砜 (7) 合成 α-碘代 β-酮砜 (7) 并用作体积试剂 (8)。一氯化碘 (1) 也被用作碘化剂,无需使用催化剂。它有两种晶型 α 和 β,其中包含以锯齿形方式排列的 ICl (1) 分子的非平面链 (9)。该化合物 (1) 具有高蒸气压,并且类似于 I2 分子,易于蒸发。虽然它已经被频繁使用,但在使用过程中存在一些危险。
-
Preparation of Disulfides by the Oxidation of Thiols Using Bromine作者:Xiaoming Wu、Reuben D. Rieke、Lishan ZhuDOI:10.1080/00397919608003879日期:1996.1Abstract Medium length aliphatic disulfides are easily synthesized via the bromine-oxidation ofthiols in the absence of solvent. Bromine can also oxidize long aliphatic and aromatic thiols to disulfides in solution. All yields are quantitative.
-
Water Accelerated Sm/TMSCl Reductive System: Debromination of<i>VIC</i>—Dibromides and Reduction of Sodium Alkyl Thiosulfates作者:Xiaoliang Xu、Ping Lu、Yongmin ZhangDOI:10.1080/00397910008087241日期:2000.6Abstract A simple and efficient method for the debromination of vic-dibromides to (E)-alkenes and reduction of sodium alkyl thiosulfates to disulfides promoted by Sm/TMSCl/H2O (trace) has been described.
-
Oxidation of Thiols to the Corresponding Symmetric Disulfides with Benzyltriphenylphosphonium Peroxodisulfate (BTPPD) Under Nonaqueous Conditions作者:Abdol R. Hajipour、Arnold E. RuohoDOI:10.1080/10426500307894日期:2003.6This article describes the preparation of benzyltriphenylphosphonium peroxodisulfate (BTPPD), an efficient and mild reagent for oxidation of a variety of aromatic and aliphatic thiols to the corresponding disulfides in refluxing acetonitrile. The experimental procedure is simple and the products are easily isolated in excellent yields.
-
Iron(III )–Ethylenediaminetetraacetic AcidMediated Oxidation of Thiols to Disulfides with MolecularOxygen†作者:Tumula Venkateshwar Rao、Bir Sain、Pappu S. Murthy、Turaga S. R. Prasada Rao、Girish C. Joshi、Ajay K. JainDOI:10.1039/a702061i日期:——Fe III âEDTA in aqueous methanoloffers a simple environmentally acceptable synthetic tool foroxidizing thiols to the corresponding disulfides by molecularoxygen in excellent yields, under mild conditions and devoid ofside reactions.
表征谱图
-
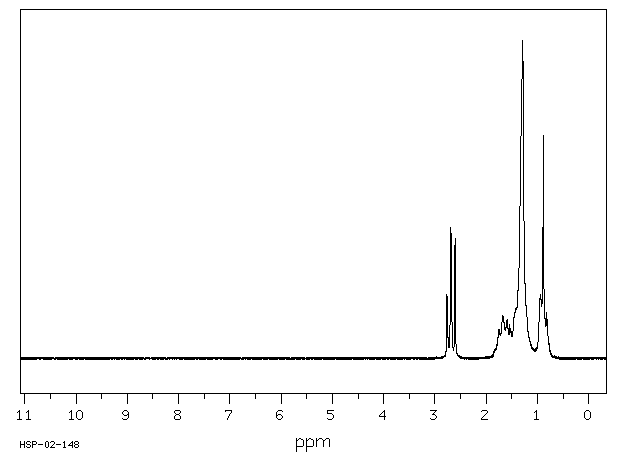
氢谱1HNMR
-
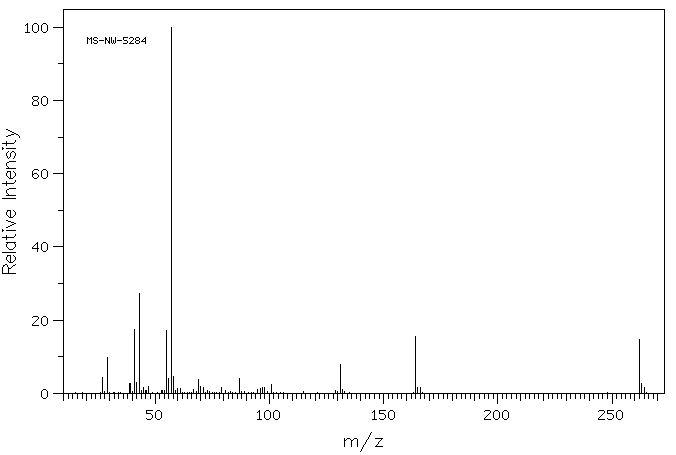
质谱MS
-
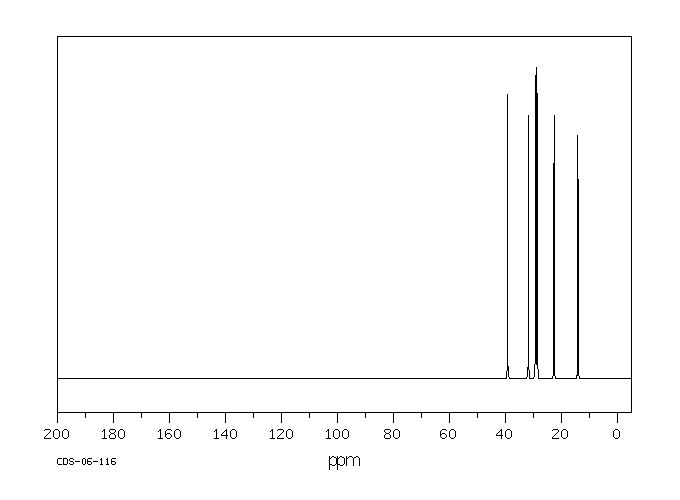
碳谱13CNMR
-
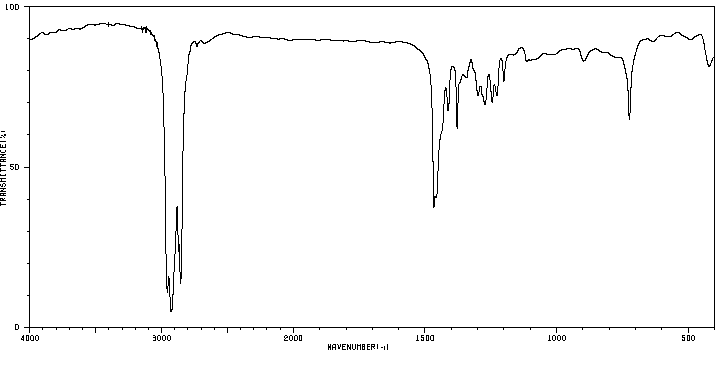
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高胱胺
胱胺
福多司坦杂质
甲基异戊基二硫醚
甲基异丙基二硫醚
甲基半胱胺
甲基丙基二硫醚
甲基丙-1-烯基二硫醚
甲基[2-甲基-1-(甲硫基)丁基]过硫化物
甲基3-甲基-1-丁烯基二硫醚
甲基-D6 二硫醚
氧化福美双
次氮基-氰基二硫基-甲烷
敌灭生
戊基甲基二硫醚
异丙基二硫醚
哌啶并,3-[2-(2-乙基苯基)肼基]-
叔丁基硫基二甲基氨基二硫代甲酸酯
叔丁基二硫
反式丙烯基丙基二硫
双羟甲基二硫化物
双正癸基二硫醚
双十六烷基二硫化物
双(十二烷基硫烷基硫代羰基)二硫化物
双(十三氟己基)二硫醚
双(三氟硫代乙酰基)二硫化物
双(2,2-二乙氧基乙基)二硫化物
双(2,2,2-三氟乙基)二硫化物
双(16-羟基十六烷基)二硫化物
双(11-羟基十一烷基)二硫化物
双(1,2-二甲基-2-氯丙基)二硫化物
原文:多(2,3-环硫烷基)二硫化物,但查不到猜测:双(2,3-环硫丙基)二硫化物
二黄原酸
二肉豆蔻基二硫醚
二硫氨磷汀
二硫化二正丁基黄原酸酯
二硫化二异丙基黄原酸酯
二硫化,二环辛基
二硫化,二(1-羰基十六烷基)
二硫代氨基甲酰二硫醚
二硫代戊酯
二甲基二硫
二甲基-13C2二硫
二环己基二硫化物
二环丙基二硫
二氯-[(甲基二硫烷基)甲氧基]甲烷
二氯-(甲基二硫烷基)甲烷
二正辛基二硫
二正庚基二硫醚
二正壬基二硫醚