二甲基氨基甲酸甲酯 | 7541-16-4
中文名称
二甲基氨基甲酸甲酯
中文别名
N,N-甲基甲酸甲酯
英文名称
methyl N,N-dimethylcarbamate
英文别名
Methyl dimethylcarbamate
CAS
7541-16-4
化学式
C4H9NO2
mdl
MFCD26794517
分子量
103.121
InChiKey
SELYJABLPLKXOY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:130-132 °C
-
密度:1.0087 g/cm3
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:29.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
包装等级:II
-
危险类别:3
-
危险性防范说明:P210,P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险品运输编号:1993
-
危险性描述:H225,H302,H315,H319,H335
-
储存条件:| 2-8°C |
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-(carbomethoxy)-α-methoxydimethylamine 76469-93-7 C5H11NO3 133.147
反应信息
-
作为反应物:参考文献:名称:ANODIC OXIDATION OF N-CARBOMETHOXYPYRROLIDINE: 2-METHOXY-N-CARBOMETHOXYPYRROLIDINE摘要:DOI:10.15227/orgsyn.063.0206
-
作为产物:描述:参考文献:名称:Geminal systems 33. Reactions of 1,1-dialkoxyureas with electrophiles and nucleophiles. synthesis of cyclic 1,1-dialkoxyureas and nh-dialkoxyamines摘要:DOI:10.1007/bf01557518
文献信息
-
一种催化甲醇转化制氨基甲酸甲酯的方法
-
Diprotonative stabilization of ring-opened carbocationic intermediates: conversion of tetrahydroisoquinoline to triarylmethanes作者:Hiroaki KurouchiDOI:10.1039/d0cc01969k日期:——Superacid-promoted conversion of tetrahydroisoquinolines to triarylmethanes via tandem reactions of C–N bond scission, Friedel–Crafts alkylation, C–O bond scission, and electrophilic aromatic amidation was developed. Dication formation was important for stabilizing the ring-opened carbocationic intermediate, which is a new role for diprotonation in reaction mechanisms.
-
Basicity of some carbonyl compounds towards iodine monochloride: experimental and theoretical study作者:Al Mokhtar Lamsabhi、Widad Bouab、M'hamed Esseffar、Manuel Alcamí、Manuel Yáñez、Jose-Luis M. AbboudDOI:10.1039/b008779n日期:——Intermolecular charge-transfer (CT) complexes between a wide range of carbonyl compounds and iodine monochloride were spectroscopically studied in the UV–visible region. Equilibrium constants and Gibbs energy changes of 1:1 charge transfer complexes were determined in CCl4 solution. The ICl basicity scale in CCl4 of the set of carbonyl derivatives included in this study is well correlated with the I2 basicity scale in the same solvent. Ab initio calculations at HF/LANL2DZ* and MP2(full)/LANL2DZ* were carried out in order to clarify the structures of these CT complexes. Two different conformations, depending on the characteristics of the substituents, may be found. In one of them the ICl moiety lies in the plane of the carbonyl group, in the other the ICl subunit is perpendicular to the CO group. The perpendicular complexes are favored by bulky substituents for which the HOMO has a clear π-character. Both kinds of complexes can be spectroscopically distinguished since they present the CT absorption at different wavelengths. In both kinds of complexes the carbonyl–ICl interaction is essentially electrostatic. The substituent effects were analyzed through the use of the Taft–Topsom model. Experimental data in solution and theoretical estimates were found to follow a good linear relationship.在紫外-可见光区域,对一系列羰基化合物与氯化碘之间的分子间电荷转移(CT)复合物进行了光谱研究。通过在CCl4溶液中测定1:1电荷转移复合物的平衡常数和吉布斯能变。研究中包含的羰基衍生物在 中的ICl碱性尺度与相同溶剂中的I2碱性尺度有很好的相关性。为了阐明这些CT复合物的结构,进行了HF/LANL2DZ*和MP2(full)/LANL2DZ*的从头计算。根据取代基的特征,可以发现两种不同的构象。在其中一种构象中,ICl部分位于羰基平面内,而在另一种构象中,ICl亚单元则垂直于CO基团。对于HOMO具有明显π特征的笨重取代基,垂直复合物更为有利。这两种类型的复合物可以通过光谱方法区分,因为它们在不同波长下呈现CT吸收。在这两种复合物中,羰基与ICl之间的相互作用主要是静电相互作用。通过使用Taft–Topsom模型分析取代基效应。实验证据与理论估计之间呈现良好的线性关系。
-
Alkali-metal-assisted transfer of the carbamate group from phosphocarbamates to alkyl halides: a new easy way to alkali-metal carbamates and to carbamate esters作者:Michele Aresta、Eugenio QuarantaDOI:10.1039/dt9920001893日期:——P(NR2)3, have been used as a source of carbamate groups in the reaction with alkyl halides, R′X, to afford carbamate esters. The reaction is mediated by alkali-metal halides, MY, and requires the presence of a suitable macrocyclic polyether (L). The overall reaction occurs in two steps: the carbamic group is first transferred to the alkali-metal cation to give a carbamate salt [ML][O2CNR2] which then reacts磷酸氨基甲酸酯P(O 2 CNR 2)x(NR 2)3 – x(R =烷基;x = 1或2),可通过将CO 2插入相应氨基膦P(NR 2)的P–N键中而容易地获得。在与烷基卤化物R'X的反应中,已经使用图3中的氨基甲酸酯作为氨基甲酸酯基团的来源,以提供氨基甲酸酯。该反应由碱金属卤化物MY介导,并且需要存在合适的大环聚醚(L)。整个反应分为两个步骤:将氨基甲酸酯基团首先转移到碱金属阳离子上,得到氨基甲酸酯盐[ML] [O 2 CNR 2然后与卤代烷反应,得到R 2 NC(O)OR'。氨基甲酸酯的产率和选择性受MY的性质的显着影响。在整个过程中的碱金属盐和大环配体在修改所述R的反应性的作用的性质的影响2 NCO 2 -阴离子中的碱金属的氨基甲酸酯进行了讨论。
-
Pentaalkylmethylguanidinium methylcarbonates – versatile precursors for the preparation of halide-free and metal-free guanidinium-based ILs作者:Benjamin Oelkers、Jörg SundermeyerDOI:10.1039/c0gc00698j日期:——methylcarbonates 6 can easily be prepared from pentaalkylguanidines 5 and dimethyl carbonate (DMC) in a sustainable solvent-free synthesis. Most of the title compounds are room temperature ionic liquids (RTILs) which provide convenient access to halide-free guanidinium-based ILs (GILs) 7via acid–base reactions and subsequent decarboxylation, similar to industrially important imidazolium methylcarbonates 1
表征谱图
-
氢谱1HNMR
-
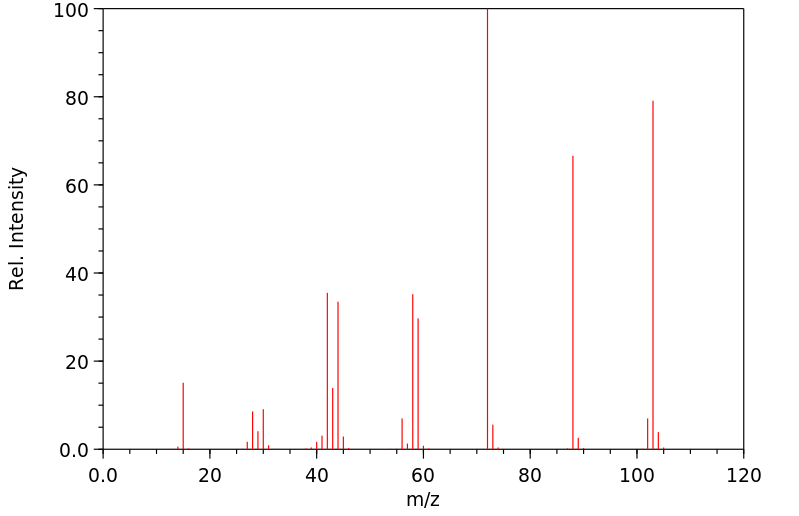
质谱MS
-
碳谱13CNMR
-
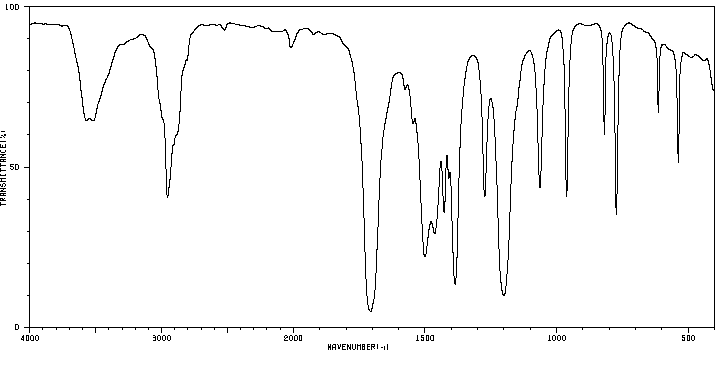
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸