二苯并-1,3a,4,6a-四氮杂并环戊二烯 | 7120-73-2
中文名称
二苯并-1,3a,4,6a-四氮杂并环戊二烯
中文别名
——
英文名称
dibenzo<1,3a,4,6a>tetraazapentalene
英文别名
5,6H-Dibenzo-1,3a,4,6a-tetraazapentalen;Dibenzo-1,3a,4,6a-tetra-azapentalen;Dibenzotetraazapentalen;5H-benzo[d]benzo[4,5][1,2,3]triazolo[2,1-a][1,2,3]triazolylium betaine;6H-Benzotriazolo[2,1-a]benzotriazol-5-ium, inner salt;benzotriazolo[2,1-a]benzotriazol-5-ium-6-ide
CAS
7120-73-2
化学式
C12H8N4
mdl
——
分子量
208.222
InChiKey
GNYDVCSYVQXVGF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:237-238 °C
-
密度:1.425 g/cm3
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:16
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:22.4
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:二苯并-1,3a,4,6a-四氮杂并环戊二烯 在 sodium azide 、 硝酸 、 氯 作用下, 以 溶剂黄146 、 二甲基亚砜 为溶剂, 反应 3.0h, 生成 3,9-Diazido-4,10-dinitrobenzotriazolo<2,1-a>benzotriazol-6-ium inner salt参考文献:名称:Reactions of Benzotriazolo[2,1-a]benzotriazole Derivatives. 1. Synthesis of New Insensitive High-Density Energetic Compounds摘要:The sequential preference of electrophilic attack on the dibenzotetraazapentalene ring system 6 has unequivocally been shown to be in the order of position 2(8) > 4(10) much greater than 1(7) and 3(9). However, nucleophilic substitution reactions with sodium azide were found to be substrate dependent. Substitution occurred at the 3(9)-position of 9 followed by elimination of hydrogen chloride to give 10 while direct substitution of azide for the 8(10)-nitro group of 2 was found to yield 13. The reactivity of the dibenzotetraazapentalene derivatives toward electrophiles and nucleophiles was exploited for the synthesis of the new heterocyclic system 14H-[1,2,5]oxadiazolo[3,4-e][1,2,5]oxadiazolo[3',4':4,5]benzotriazolo[2,1-alpha]benzotriazol-6-ium inner salt 1,8-dioxide (11). From this study the first of a new class of insensitive energetic materials 4 has been synthesized in a straightforward fashion from 2.DOI:10.1021/jo00124a024
-
作为产物:描述:2,2'-dinitrenoazobenzene 以 gaseous matrix 为溶剂, 生成 二苯并-1,3a,4,6a-四氮杂并环戊二烯参考文献:名称:Intermediates in photolyses of 2,2′-diazidoazobenzene and 2-azidoazobenzene in low-temperature matrices摘要:The photolyses of 2,2'-diazidoazobenzene (1) and 2-azidoazobenzene (4) were investigated in argon and 2-methyltetrahydrofuran (MTHF) matrices at cryogenic temperature. Nitrene and dinitrene were observed in the photolysis of 1 in an MTHF matrix by electron spin resonance (ESR). The dinitrene in an argon matrix was also observed by ultraviolet-visible (UV-vis) and Fourier transform infrared (FT-IR) spectroscopies. UV absorption bands at 430 and 460 nm and IR peak at 1263 cm(-1) were assigned to the dinitrene. Furthermore, mononitrene as an intermediate of the photolysis of 4 was confirmed by UV-vis and FT-IR spectroscopies in an argon matrix. An IR band at 1225 cm(-1) of mononitrene was observed. (C) 1998 Elsevier Science B.V. All rights reserved.DOI:10.1016/s1386-1425(97)00231-x
表征谱图
-
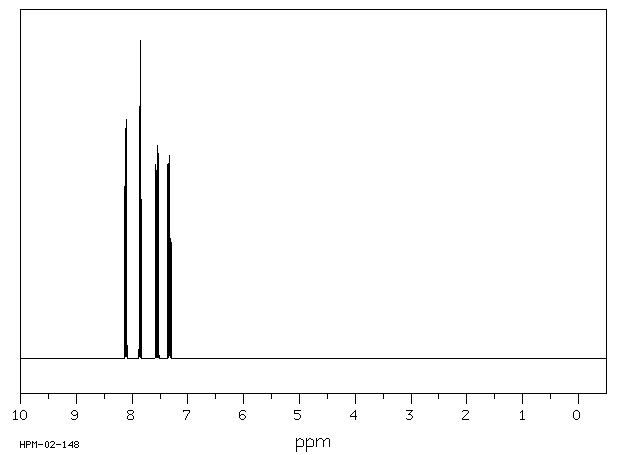
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿立必利
试剂4,7-Bis(5-bromo-2-thienyl)-5,6-difluoro-2-(2-hexyldecyl)-2H-benzotriazole
苯并三氮唑-N,N,N',N'-四甲基脲六氟磷酸盐
苯并三氮唑-5-甲酸乙酯
苯并三氮唑-1-基吡咯烷-1-基甲硫酮
苯并三唑-D4
苯并三唑-5(6)-甲磺酸
苯并三唑-1-羧硫代酸烯丙基酰胺
苯并三唑-1-羧硫代酸(furan-2-ylmethyl)酰胺
苯并三唑-1-羧硫代酸 2-噻唑基酰胺
苯并三唑-1-碳酰氯
苯并三唑-1-甲酰胺
苯并三唑-1-基甲基-环戊基-胺
苯并三唑-1-基氧基-三(二甲基氨基)鏻
苯并三唑-1-基丙-2-烯基碳酸酯
苯并三唑-1-基(四氢-1H-1,4-恶嗪-4-基)甲亚胺
苯并三唑-1-亚氨基丙二酸二乙酯
羟基苯并三氮唑活性酰胺
羟基苯并三氮唑活性酯
羟基苯并三唑
甲基4-氨基-1H-苯并三唑-6-羧酸酯
甲基1-乙基-1H-苯并三唑-6-羧酸酯
氯化1-(1H-苯并三唑-1-基甲基)-3-甲基哌啶正离子
曲苯的醇
异乔木萜醇乙酸酯
多肽试剂TCTU
四丁基苯并三唑盐
吡唑并苯并[1,2-a]三唑
双(1H-苯并三唑-5-胺)硫酸盐
双(1H-苯并三唑-5-胺)硫酸盐
双(1-苯并[d]三唑)碳酸酯
双(1-(苯并三唑-1-基)-2-甲基丙基)胺
卡特缩合剂
伏罗唑
伏罗唑
伏氯唑
二苯并-1,3a,4,6a-四氮杂并环戊二烯
二(苯并三唑-1-基甲基)胺
二(苯并三唑-1-基氧基)-甲基膦
二(苯并三唑-1-基)甲亚胺
二(1H-苯并三唑-1-基)甲酮
二(1H-苯并三唑-1-基)亚砜
二(1-苯并三唑基)草酸酯
二(1-苯并三唑基)甲硫酮
乙醇,2-(2-噻唑基甲氧基)-
乙酮,2-[(3-甲基-2-吡啶基)氨基]-1-苯基-
三环唑
三氮唑杂质1
三-(1-苯并三唑基)甲烷
三(苯并三唑-1-基甲基)胺