亚乙基硫脲 | 96-45-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:196-200 °C(lit.)
-
密度:1.41~1.45
-
闪点:252 °C
-
溶解度:8克/升
-
沸点:240°C (1010 hPa)
-
LogP:-0.67 at 20℃
-
物理描述:Ethylene thiourea appears as white to pale green crystals or an off-white solid. Odorless when pure, but technical product may have an amine odor. (NTP, 1992)
-
颜色/状态:Needles, prisms from alcohol or amyl alcohol
-
气味:Faint, amine odor
-
蒸汽压力:2.02X10-6 mm Hg at 25 °C (est)
-
分解:When heated to decomposition it emits very toxic fumes of /nitroxides/ and /sulfoxides/.
-
电离电位:8.15 eV
-
气味阈值:Odorless
-
稳定性/保质期:
在常温常压下,该物质性质稳定。
计算性质
-
辛醇/水分配系数(LogP):-0.7
-
重原子数:6
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:56.2
-
氢给体数:2
-
氢受体数:1
ADMET
安全信息
-
危险等级:6.1(b)
-
危险品标志:T
-
安全说明:S45,S53
-
危险类别码:R22,R61
-
WGK Germany:2
-
海关编码:29332990
-
危险品运输编号:2811
-
危险类别:6.1(b)
-
RTECS号:NI9625000
-
包装等级:III
-
危险标志:GHS07,GHS08
-
危险性描述:H302,H351,H360D,H372
-
危险性防范说明:P201,P281,P308 + P313
-
储存条件:使用木桶或编织袋进行包装,并储存在干燥的库房中。请注意防潮,同时要远离火源,因为本品属于可燃物品。
SDS
: 2-硫醇基咪唑啉
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
眼刺激 (类别2B)
致畸性 (类别1B)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H302 吞咽有害。
H320 造成眼刺激。
H360 可能对生育能力或胎儿造成伤害。
警告申明
预防
P201 在使用前获取特别指示。
P202 在读懂所有安全防范措施之前切勿操作。
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P281 使用所需的个人防护设备。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P308 + P313 如接触到或有疑虑:求医/ 就诊。
P330 漱口。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
储存
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
只限于专业使用者。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C3H6N2S
分子式
: 102.16 g/mol
分子量
组分 浓度或浓度范围
2-Imidazolidinethione
-
CAS 号 96-45-7
EC-编号 202-506-9
索引编号 613-039-00-9
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 硫氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境保护措施
在确保安全的前提下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。避免曝露:使用前需要获得专门的指导。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
沉浸保护
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: > 480 min
测试过的物质Dermatril® ( Z677272, 规格 M)
飞溅保护
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: > 30 min
测试过的物质Dermatril® ( Z677272, 规格 M)
0, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不 同于EN
374规定的条件下应用,请与EC批准的手套的供应 商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N100型(US
)或P3型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
无数据资料
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 1,832 mg/kg
皮肤刺激或腐蚀
眼睛刺激或腐蚀
眼睛 - 兔子 - 轻度的眼睛刺激
呼吸道或皮肤过敏
长期或反复接触导致个别人过敏反应
生殖细胞突变性
细胞突变性-体外试验 - 老鼠 - 淋巴细胞
微生物突变
细胞突变性-体外试验 - 老鼠 - 鼠伤寒沙门氏菌
宿主介入的测试
细胞突变性-体外试验 - 仓鼠 - 肾
形态变形
细胞突变性-体内试验 - 大鼠 - 经口
细胞发生分析
细胞突变性-体内试验 - 老鼠 - 腹膜内的
DNA损伤
致癌性
致癌性 - 大鼠 - 经口
肿瘤发生:符合RTECS标准的致癌性。 肺,胸,或者呼吸系统:肿瘤 内分泌的:甲状腺肿瘤
致癌性 - 老鼠 - 经口
肿瘤发生:符合RTECS标准的致癌性。 肝脏:肿瘤 内分泌的:甲状腺肿瘤
致癌性 - 大鼠 - 经口
肿瘤发生:符合RTECS标准的致癌性。 内分泌的:甲状腺肿瘤 肿瘤发生作用:睾丸肿瘤。
致癌性 - 大鼠 - 经口
肿瘤发生:符合RTECS标准的可疑致癌试剂。 肝脏:肿瘤
IARC:
3 - 第3组:未被分类为对人类致癌 (2-Imidazolidinethione)
生殖毒性
假设有人类生殖毒性
致畸性 - 大鼠 - 经口
对新生儿的影响:存活率(例如#第4天存活率每#出生成活数)。
对新生儿的影响:离乳指数或哺乳指数(例如#断乳后成活每#第4天成活数)。
致畸性 - 大鼠 - 皮肤
母体效应:分娩。 对新生儿的影响:死婴。 对新生儿的影响:生长统计数据(例如体重增长的减少)。
致畸性 - 大鼠 - 腹膜内的
对生殖的影响:数量少(例如#每胎产仔;出生前测定)。 特定发育异常:中枢神经系统。
对新生儿的影响:存活率(例如#第4天存活率每#出生成活数)。
致畸性 - 老鼠 - 经口
对生殖的影响:胚胎植入后死亡率(例如总着床胚胎数中死亡和/或被再吸收的胚胎数)。
对胚胎或胎儿的影响:胎儿死亡。 特定发育异常:肌肉骨骼系统。
发育毒性 - 兔子 - 经口
特定发育异常:中枢神经系统。
发育毒性 - 大鼠 - 皮下的
对胚胎或胎儿的影响:胎儿毒性(死亡除外,例如矮小胎儿)。 特定发育异常:中枢神经系统。
特定发育异常:颅面(包括鼻和舌)。
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 造成眼刺激。
附加说明
化学物质毒性作用登记: NI9625000
模块 12. 生态学资料
12.1 生态毒性
对鱼类的毒性 半数致死浓度(LC50) - PoECilia reticulata (红鳉) - 7,500 mg/l - 96 h
对水蚤和其他水生无脊 半数致死浓度(LC50) - Daphnia magna (大型蚤) - 26.4 mg/l - 48 h
椎动物的毒性
对藻类的毒性 生长抑制 半致死有效浓度(EC50) - Chlorella pyrenoidOSa - 6,600 mg/l - 96 h
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
亚乙基硫脲是一种咪唑啉类硫化促进剂,纯品为白色针状结晶,微溶于水,具有轻微的氨臭。它曾广泛用于氯丁橡胶及其他橡胶的固化,并被少量用于生产杀虫剂。但由于对人体的危害,目前已基本被淘汰。
毒性亚乙基硫脲的LD50值为1832 mg/kg(大鼠口服),属于低毒化学物质。关于其环境浓度限制目前没有统一标准,美国某些州规定空气中限制为0~0.7 μg/m³,EPA建议水中限制为0.44 μg/L。
危害亚乙基硫脲被国际癌症研究机构(IARC)列为第三类致癌物——对人类的致癌性不确定(曾为2B类),但在动物实验中显示出致癌和致畸性的阳性结果。在小鼠实验中,它引起了甲状腺滤泡细胞癌、垂体前叶腺肿瘤及肝癌;在大鼠中则导致甲状腺上皮细胞癌。此外,亚乙基硫脲与硫脲、丙硫氧嘧啶等抗甲状腺物质类似,会导致人类甲状腺肿大,并引起黏液水肿和胎儿畸形。
应用 合成乙烯中的应用亚乙基硫脲在合成乙烯中具有重要作用。乙烯是一种重要的有机化工原料,在化学工业中有广泛的应用。制备乙烯的方法包括烷铝和锰碳催化剂等,但这些方法存在环境污染、成本高等问题。相比之下,乙烯硫脲在这方面具有独特优势。在实验室中,可以使用乙烯硫脲与碘化氢反应制备乙烯。这种反应能降低反应条件,减少环境污染和降低成本。
亚乙基硫脲还可以与其他化合物缩合形成新的含硫杂环化合物,具有研究价值和应用前景。
化学性质亚乙基硫脲为白色至淡绿色晶体,具有微弱的氨臭。它在冷水中微溶,在热水中易溶,并在室温下轻微溶解于乙醇、甲醇、醋酸和汽油中。但不溶于丙酮、乙醚和氯仿。
用途亚乙基硫脲作为咪唑啉类硫化促进剂,可用于氯丁橡胶、氯醇橡胶及氯化聚乙烯等材料的加工。特别适合用于非硫化体系中氯丁胺的合成促进剂。在100-500℃下,采用适当的配合体系,可实现快速最佳硫化,并确保操作安全。其硫化制品具有高强度和低永久压缩变形的特点,尤其适用于W型(即54-1型)氯丁橡胶的非硫化体系。该产品通常与氧化锌和氧化镁配合使用,广泛应用于工业制品、被覆电线及鞋衣等氯丁橡胶制品。
此外,亚乙基硫脲还可作为硫酸铜的辅助光亮剂,并用于精细化学领域的中间体制造,包括抗氧剂、杀虫剂、杀真菌剂、农药、食品防腐剂以及镀铜光亮剂等。其生产方法通常涉及乙二胺与二硫化碳反应,生成乙烯基二硫代氨基甲酸盐后进一步环化生成乙烯硫脲。
近年来,它还被用于饮用水污染物的检测和处理,作为一种候选名单3 (CCL 3)化合物受到美国环境保护署(EPA)的关注。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— N-(β-Aminoethyl)-thioharnstoff 3685-60-7 C3H9N3S 119.191
反应信息
-
作为反应物:描述:参考文献:名称:Crystal structures of polyiodide salts and molecular complexes. 7. Interaction of thiones with molecular diiodine. The crystal structures of dithizone-diiodine, ethylenethiourea-bis(diiodine), bis(ethylenethiourea)-tris(diiodine), bis(dithizone)-heptakis(diiodine), and 1-(1-imidazolin-2-yl)-2-thioxoimidazolidinium triiodide-(ethylenethiourea-diiodine)摘要:DOI:10.1021/ja00320a024
-
作为产物:描述:N-(2-aminoethyl)formamide 在 sulfur 作用下, 生成 亚乙基硫脲参考文献:名称:环状硫脲。摘要:DOI:10.1021/ja01211a509
-
作为试剂:参考文献:名称:通过未活化炔烃与1,3-二卤代-5,5-二甲基乙内酰脲的催化级联反应,水控选择性制备[小α]-单或[小α] [小[伯或分钟]-二卤代酮摘要:已经开发了通过水控制的化学发散和区域特异性级联反应选择性合成[小α]-单或[小α],[小α] [伯或分钟]-二卤代酮的有效方案。DOI:10.1039/c7gc00283a
文献信息
-
Melanocortin-4 receptor binding compounds and methods of use thereof申请人:Millennium Pharmaceuticals, Inc.公开号:US20040082779A1公开(公告)日:2004-04-29Provided are MC4-R binding compounds of the formula XVII: 1 wherein L 2 is a linker group, and P 1 , P 2 , P 3 , P 4 , Z 1 , Z 2 , Z 3 , Z 4 , Z 5 , t, s, and R are as described in the specification. Methods of using the compounds to treat MC4-R associated disorders, such as disorders associated with weight loss, are also provided.
-
Structures of Addition Products of Acetylenedicarboxylic Acid Esters with Various Dinucleophiles. An application of C, H-spin-coupling constants作者:Ulrich Vögeli、Wolfgang von Philipsborn、Kuppuswamy Nagarajan、Mohan D. NairDOI:10.1002/hlca.19780610207日期:1978.3.8Heterocyclic compounds obtained by addition of acetylenedicarboxylic acid esters to thioureas, cyclic amidines and o-difunctionalized aromatic systems have been studied by 13C-NMR. In particular, C, H-spin-coupling constants over two and three bonds were used to differentiate between the various constitutional isomers and to establish the configuration of trisubstituted exocyclic C, C-double bonds
-
Palladium(0)-Catalyzed, Copper(I)-Mediated Coupling of Boronic Acids with Cyclic Thioamides. Selective Carbon−Carbon Bond Formation for the Functionalization of Heterocycles<sup>†</sup>作者:Hana Prokopcová、C. Oliver KappeDOI:10.1021/jo070408f日期:2007.6.1Employing controlled microwave irradiation at 100 °C, most cross-couplings can be completed within 2 h and proceed in high yields. An advantage of using thioamides as starting materials is the fact that the system can be tuned to an alternative carbon−sulfur cross-coupling pathway by changing to stoichiometric copper(II) under oxidative conditions. Both types of thioamide cross-couplings are orthogonal to
-
[EN] cGAS ANTAGONIST COMPOUNDS<br/>[FR] COMPOSÉS ANTAGONISTES DU CGAS申请人:IMMUNE SENSOR LLC公开号:WO2017176812A1公开(公告)日:2017-10-12Disclosed are novel compounds of Formula (I) that are cGAS antagonists, methods of preparation of the compounds, pharmaceutical compositions comprising the compounds, and their use in medical therapy.揭示了一种新型化合物的化学式(I),这些化合物是cGAS拮抗剂,涉及到这些化合物的制备方法、包含这些化合物的药物组合物,以及它们在医学治疗中的应用。
-
BBI608衍生物及其制备与用途
表征谱图
-
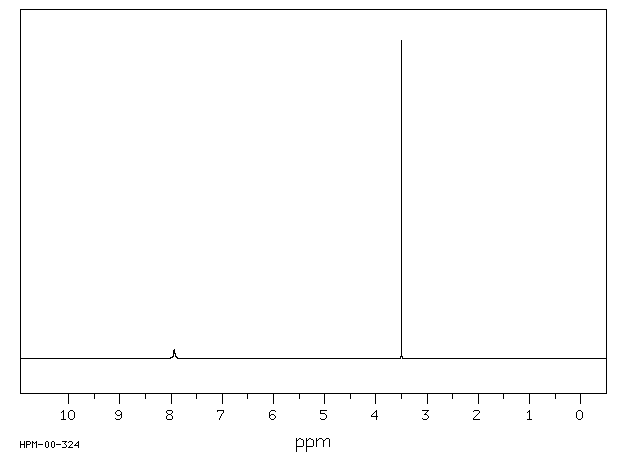
氢谱1HNMR
-
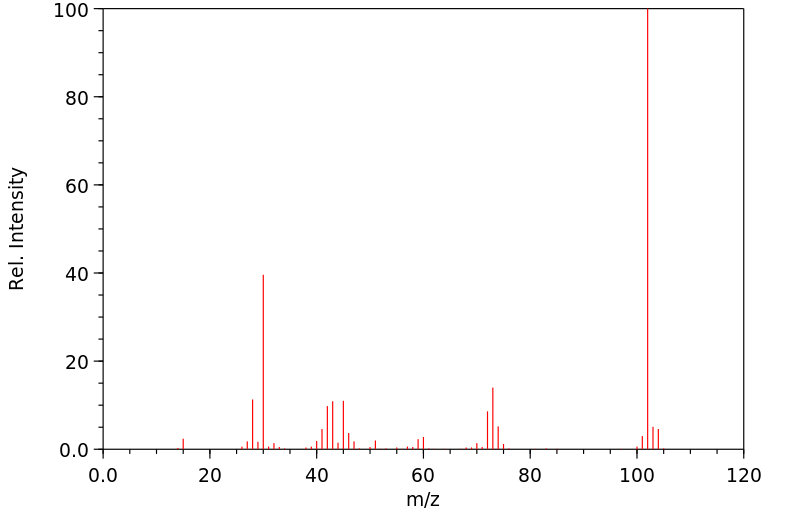
质谱MS
-
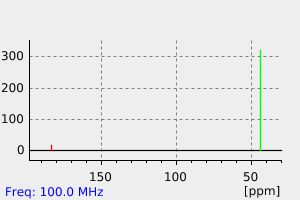
碳谱13CNMR
-
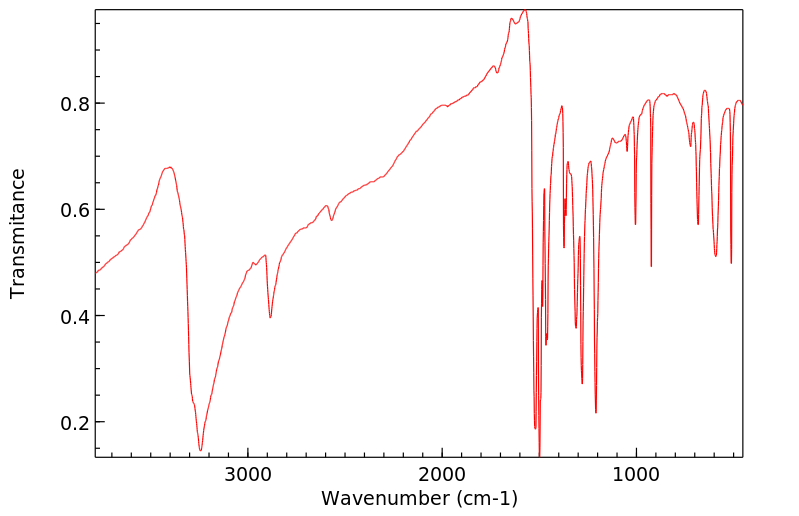
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息