优降宁 | 306-07-0
中文名称
优降宁
中文别名
巴吉林,优降宁;N-甲基-N-炔丙基苄胺盐酸盐;盐酸帕吉林;巴吉林盐酸盐;丙炔甲基苄胺;帕吉林
英文名称
pargyline hydrochloride
英文别名
N-benzyl-N-methylprop-2-yn-1-amine hydrochloride;N-methyl-N-benzylpropargylamine hydrochloride;N-methyl-N-propargylbenzylamine hydrochloride;pargyline HCl;pargyline hydrochoride;N-benzyl-N-methylprop-2-yn-1-amine;hydron;chloride
CAS
306-07-0
化学式
C11H13N*ClH
mdl
MFCD00012492
分子量
195.692
InChiKey
BCXCABRDBBWWGY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:160-163 °C(lit.)
-
溶解度:乙醇中≥33.55 mg/mL;水中≥51.6 mg/mL; ≥7.55 mg/mL,溶于 DMSO
-
碰撞截面:134.9 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
稳定性/保质期:
如果按照规定使用和储存,则不会发生分解,也不存在已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.14
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.272
-
拓扑面积:3.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:6.1(b)
-
危险品标志:Xn
-
安全说明:S22,S24/25
-
危险类别码:R22
-
WGK Germany:3
-
包装等级:III
-
危险类别:6.1(b)
-
危险标志:GHS06
-
危险品运输编号:UN 2811 6.1/PG 3
-
危险性描述:H301
-
危险性防范说明:P301 + P310
-
储存条件:密封储存,并在-20 °C下保存。
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : Pargyline hydrochloride
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 306-07-0
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, Oral (Category 3), H301
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Xn Harmful R22
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Danger
Hazard statement(s)
H301 Toxic if swallowed.
Precautionary statement(s)
P301 + P310 IF SWALLOWED: Immediately call a POISON CENTER or doctor/
physician.
Supplemental Hazard none
Statements
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Chemical characterization : Natural product
Synonyms : N-Methyl-N-propargylbenzylamine
N-Methyl-N-(2-propynyl)benzylamine
Formula : C11H13N · HCl
Molecular Weight : 195,69 g/mol
CAS-No. : 306-07-0
EC-No. : 206-175-1
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
Pargyline hydrochloride
CAS-No. 306-07-0 Acute Tox. 3; H301 <= 100 %
EC-No. 206-175-1
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Take victim immediately to hospital. Consult a physician.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx), Hydrogen chloride gas
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Wear respiratory protection. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
For personal protection see section 8.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Recommended storage temperature: -20 °C
Specific end use(s)
A part from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Avoid contact with skin, eyes and clothing. Wash hands before breaks and immediately after handling
the product.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Full contact
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: 480 min
Material tested:Dermatril® (KCL 740 / Z677272, Size M)
Splash contact
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: 480 min
Material tested:Dermatril® (KCL 740 / Z677272, Size M)
data source: KCL GmbH, D-36124 Eichenzell, phone +49 (0)6659 87300, test method: EN374
If used in solution, or mixed with other substances, and under conditions which differ from EN 374,
contact the supplier of the CE approved gloves. This recommendation is advisory only and must
be evaluated by an industrial hygienist and safety officer familiar with the specific situation of
anticipated use by our customers. It should not be construed as offering an approval for any
specific use scenario.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face particle
respirator type N99 (US) or type P2 (EN 143) respirator cartridges as a backup to engineering
controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use
respirators and components tested and approved under appropriate government standards such
as NIOSH (US) or CEN (EU).
Control of environmental exposure
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: crystalline
Colour: white
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 160 - 163 °C - lit.
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility 50 g/l
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Chloroformates, Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
LD50 Oral - rat - 250 mg/kg
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
Reproductive toxicity - rat - Intraperitoneal
Paternal Effects: Other effects on male.
Reproductive toxicity - mouse - Subcutaneous
Effects on Fertility: Other measures of fertility
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: DP6650000
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: 2811 IMDG: 2811 IATA: 2811
UN proper shipping name
ADR/RID: TOXIC SOLID, ORGANIC, N.O.S. (Pargyline hydrochloride)
IMDG: TOXIC SOLID, ORGANIC, N.O.S. (Pargyline hydrochloride)
IATA: Toxic solid, organic, n.o.s. (Pargyline hydrochloride)
Transport hazard class(es)
ADR/RID: 6.1 IMDG: 6.1 IATA: 6.1
Packaging group
ADR/RID: III IMDG: III IATA: III
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
生物活性
体外研究
细胞周期分析
凋亡分析
蛋白质表达分析
体内研究
Pargyline hydrochloride 是一种不可逆的单胺氧化酶 (MAO) 抑制剂,对 MAO-A 和 MAO-B 的 Ki 值分别为 13 μM 和 0.5 μM。该化合物具有降压和抗癌活性。
靶点| Target | Value |
|---|---|
| MAO-B (Cell-free assay) | 0.5 μM(Ki) |
| MAO-A (Cell-free assay) | 13 μM(Ki) |
- Pargyline 处理(浓度:0.5-2 mM;时间:24-120 小时;细胞系:LNCaP-LN3)抑制前列腺癌细胞的增殖,表现出时间和剂量依赖性。
- Pargyline 处理(浓度:0.5-2 mM;时间:24-48 小时;细胞系:LNCaP-LN3)导致 G1 期比例增加和 S 期比例减少,具有剂量依赖性。
- Pargyline 处理(浓度:0.5 mM;时间:24 小时;细胞系:LNCaP-LN3)诱导凋亡细胞增多。
- Pargyline 处理(浓度:2 mM;时间:48 小时;细胞系:LNCaP-LN3)导致细胞中细胞色素 c 增加和 caspase-3 减少,但未改变 BCL-2 表达。
| 实验参数 | 详情 |
|---|---|
| 细胞系 | LNCaP-LN3细胞 |
| 浓度 | 0.5 mM, 1 mM, 1.5 mM 或 2 mM |
| 孵育时间 | 24 小时, 48 小时, 72 小时, 96 小时或 120 小时 |
| 结果 | 抑制前列腺癌细胞的增殖,表现出时间和剂量依赖性 |
| 实验参数 | 详情 |
|---|---|
| 细胞系 | LNCaP-LN3细胞 |
| 浓度 | 0.5 mM, 2 mM |
| 孵育时间 | 24 小时, 48 小时 |
| 结果 | S期细胞比例减少,G1期细胞比例增加 |
| 实验参数 | 详情 |
|---|---|
| 细胞系 | LNCaP-LN3细胞 |
| 浓度 | 0.5 mM |
| 孵育时间 | 24 小时 |
| 结果 | 引起细胞色素 c 增加和 caspase-3 减少 |
| 实验参数 | 详情 |
|---|---|
| 细胞系 | LNCaP-LN3细胞 |
| 浓度 | 2 mM |
| 孵育时间 | 48 小时 |
| 结果 | 引起细胞色素 c 增加和 caspase-3 减少 |
- Pargyline(剂量:10 mg/kg;静脉注射)在未麻醉的自发性高血压大鼠(SHR)中引起适度且持久的血压下降(约20 mm Hg,持续48小时),但对正常血压的大鼠无效。
- 低剂量 Pargyline (200 μg 直接脑内注射)降低动脉血压。Pargyline 对 SHR 的降压作用可能是由于神经递质去甲肾上腺素在大脑中的积累。
具有明显的降压作用,是单胺氧化酶 (MAO-B) 的选择性抑制剂。
类别有毒物品
毒性分级高毒
急性毒性- 口服 - 大鼠 LD50: 142 mg/kg; 小鼠 LD50: 680 mg/kg
可燃;燃烧产生有毒氮氧化物和氯化氢烟雾;药物副作用:影响心理状态;影响液体吸收
储运特性通风低温干燥;与库房食品原料分开存放
灭火剂反应信息
-
作为反应物:描述:5-iodo-2-tert-butylindolizine 、 优降宁 在 sodium 、 bis-triphenylphosphine-palladium(II) chloride 、 copper(l) iodide 作用下, 以 甲醇 、 乙腈 为溶剂, 反应 0.75h, 以86%的产率得到N-benzyl-3-(2-tert-butylindolizin-5-yl)-N-methylprop-2-yn-1-amine参考文献:名称:吲哚嗪环的Sonogashira反应摘要:摘要 在二氯双(三苯基膦)钯,碘化铜(I)和三乙胺在乙腈中的存在下,使2-叔丁基-5-碘代吲哚嗪与乙炔进行Sonogashira反应,以高收率得到相应的5-乙炔基吲哚并咪唑。5-碘-2-苯基吲哚嗪和5-溴-2-叔丁基吲哚嗪不进行反应。X射线表征了几种结构。5-乙炔基吲哚并没有环化生成环[3.2.2]嗪。 在二氯双(三苯基膦)钯,碘化铜(I)和三乙胺在乙腈中的存在下,使2-叔丁基-5-碘代吲哚嗪与乙炔进行Sonogashira反应,以高收率得到相应的5-乙炔基吲哚并咪唑。5-碘-2-苯基吲哚嗪和5-溴-2-叔丁基吲哚嗪不进行反应。X射线表征了几种结构。5-乙炔基吲哚并没有环化生成环[3.2.2]嗪。DOI:10.1055/s-0034-1378861
-
作为产物:参考文献:名称:LAMBERT, S. J.;KABALKA, G. W.;KNAPP, F. F. (RUSS) (JR);SRIVASTAVA, P. C., J. ORG. CHEM., 56,(1991) N1, C. 3707-3711摘要:DOI:
文献信息
-
[EN] R(-)-2-METHOXY-11-HYDROXYAPORPHINE AND DERIVATIVES THEREOF<br/>[FR] R(-)-2-MÉTHOXY-11-HYDROXYAPORPHINE ET SES DÉRIVÉS
-
[EN] AZASPIROALKENE AND AZASPIROALKANE COMPOUNDS WITH NICOTINIC CHOLINERGIC RECEPTOR ACTIVITY<br/>[FR] COMPOSES D'AZASPIROALCENE ET D'AZASPIROALCANE A ACTIVITE DE RECEPTEURS NICOTINIQUES CHOLINERGIQUES申请人:TARGACEPT INC公开号:WO2006034089A1公开(公告)日:2006-03-30Compounds, pharmaceutical compositions including the compounds, and methods of preparation and use thereof are disclosed. The compounds are N-aryl or heteroaryl azaspiroalkene/alkane compounds, prodrugs or metabolites of these compounds, or pharmaceutically acceptable salts thereof. The aryl group can be a phenyl ring or a five- or six-membered heterocyclic ring (heteroaryl). The compounds and compositions can be used to treat and/or prevent a wide variety of conditions or disorders, particularly those disorders characterized by dysfunction of nicotinic cholinergic neurotransmission, including disorders involving neuromodulation of neurotransmitter release, such as dopamine release. CNS disorders, which are characterized by an alteration in normal neurotransmitter release, are another example of disorders that can be treated and/or prevented. The compounds and compositions can also be used to alleviate pain. The compounds can: (i) alter the number of nicotinic cholinergic receptors of the brain of the patient, (ii) exhibit neuroprotective effects and (iii) when employed in effective amounts, not result in appreciable adverse side effects (e.g., side effects such as significant increases in blood pressure and heart rate, significant negative effects upon the gastro-intestinal tract, and significant effects upon skeletal muscle).揭示了化合物、包括这些化合物的药物组合物以及其制备和使用方法。这些化合物是N-芳基或杂环芳基氮杂螺环烯/烷化合物,这些化合物的前药或代谢物,或其药用可接受的盐。芳基可以是苯环或五元或六元杂环环(杂环芳基)。这些化合物和组合物可用于治疗和/或预防各种各样的疾病或紊乱,特别是那些以胆碱能神经递质功能障碍为特征的疾病,包括涉及神经递质释放的神经调节紊乱,如多巴胺释放的疾病。中枢神经系统疾病,其特征是正常神经递质释放的改变,是可以治疗和/或预防的另一个例子。这些化合物和组合物也可用于缓解疼痛。这些化合物可以:(i)改变患者大脑中的胆碱能受体数量,(ii)表现出神经保护作用,以及(iii)在有效剂量下使用时,不会导致明显的不良副作用(例如,明显增加血压和心率、对胃肠道产生明显负面影响,以及对骨骼肌产生明显影响)。
-
Sulfonyl-containing 2,3-diarylindole compounds, methods for making same, and methods of use thereof申请人:——公开号:US20040058977A1公开(公告)日:2004-03-25The present invention relates to sulfonyl-containing 2,3-diarylindole, especially to new compounds of general Formula, to a preparation method for their preparation, to pharmaceutical compositions containing said compound, and to the medical use thereof in the treatment of diseases relating to the inhibition of cyclooxygenase-2 (COX-2).本发明涉及含砜基的2,3-二芳基吲哚,特别涉及一般式的新化合物,其制备方法,含有该化合物的药物组合物,以及在治疗与环氧合酶-2(COX-2)抑制有关的疾病中的医疗用途。
-
AMINE COMPOUND FOR INHIBITING SSAO / VAP-1 AND USE THEREOF申请人:SUNSHINE LAKE PHARMA CO., LTD.公开号:US20200377461A1公开(公告)日:2020-12-03An amine compound serving as a semicarbazide-sensitive amine oxidase (SSAO) and/or vascular adhesion protein-1 (VAP-1) inhibitor, a pharmaceutical composition, and an application thereof in medicines that can be used for treating inflammation and/or inflammation related diseases, diabetes and/or a disease related diabetes, psychiatric disorder, ischemic disease, vascular disease, fibrosis, or tissue transplant rejection.
-
ADENOSINE A2A RECEPTOR ANTAGONISTS申请人:Moorman Allan R.公开号:US20080242672A1公开(公告)日:2008-10-02The present invention provides compounds of the formula wherein R 1 and R 2 have meaning as defined herein in the specification. The compounds of formula (I) are adenosine A 2A receptor antagonists and, thus, may be employed for the treatment of conditions and diseases mediated by the adenosine A 2A receptor activity. Such conditions include, but are not limited to, diseases of the central nervous system such as depression, cognitive function diseases and neurodegenerative diseases such as Parkinson's disease, senile dementia as in Alzheimer's disease or psychoses and stroke. The compounds of the present invention may also be employed for the treatment of attention related disorders such as attention deficit disorder (ADD) and attention deficit hyperactivity disorder (ADHD), extra pyramidal syndrome, e.g., dystonia, akathisia, pseudoparkinsonism and tardive dyskinesia, and disorders of abnormal movement such as restless leg syndrome (RLS) and periodic limb movement in sleep (PLMS); cirrhosis, and fibrosis and fatty liver; dermal fibrosis in diseases such as scleroderma; and the mitigation of addictive behavior. In particular, the compounds of the present invention may be employed to improve motor-impairment due to neurodegenerative diseases such as Parkinson's disease.本发明提供了以下式的化合物 其中R 1 和R 2 的含义如规范中所定义。式(I)的化合物是腺苷A 2A 受体拮抗剂,因此可用于治疗由腺苷A 2A 受体活性介导的疾病和病症。这些疾病包括但不限于中枢神经系统疾病,如抑郁症、认知功能疾病和神经退行性疾病,如帕金森病、老年性痴呆症、精神病和中风。本发明的化合物还可用于治疗注意力相关障碍,如注意力缺陷障碍(ADD)和注意力缺陷多动障碍(ADHD),额外锥体综合征,例如肌张力障碍、不安坐立、假性帕金森症和迟发性运动障碍,以及异常运动障碍,如不宁腿综合征(RLS)和睡眠中期肢体运动(PLMS);肝硬化、纤维化和脂肪肝;在硬皮病等疾病中的皮肤纤维化;以及对成瘾行为的缓解。特别是,本发明的化合物可用于改善由帕金森病等神经退行性疾病引起的运动障碍。
表征谱图
-
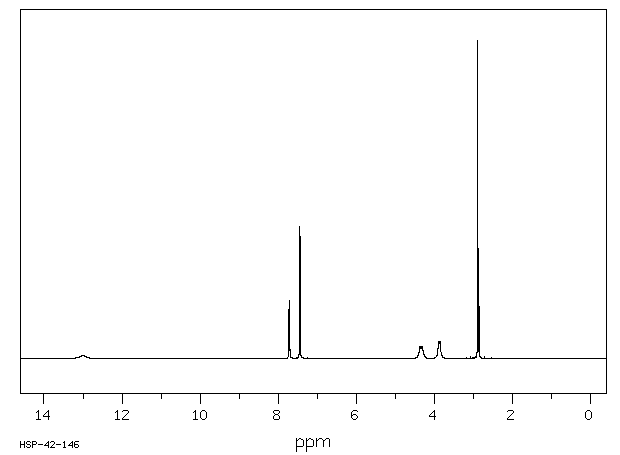
氢谱1HNMR
-
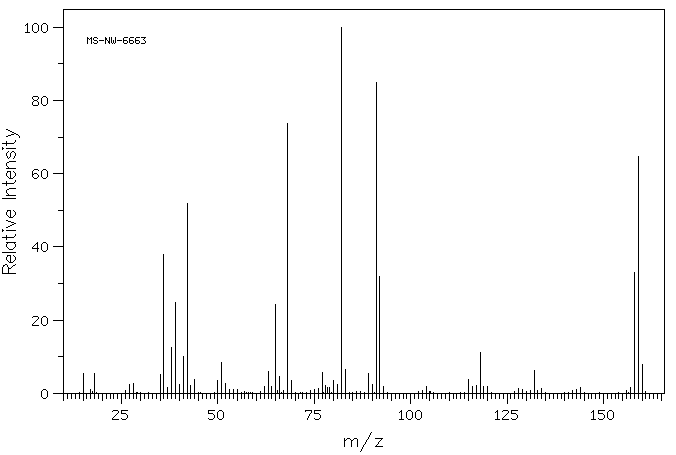
质谱MS
-
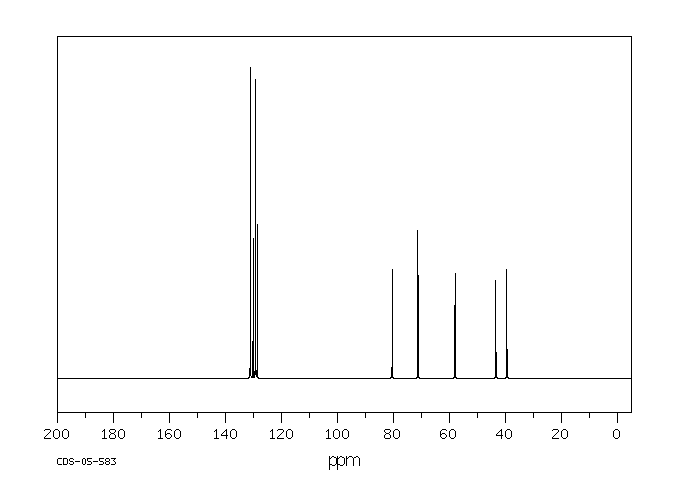
碳谱13CNMR
-
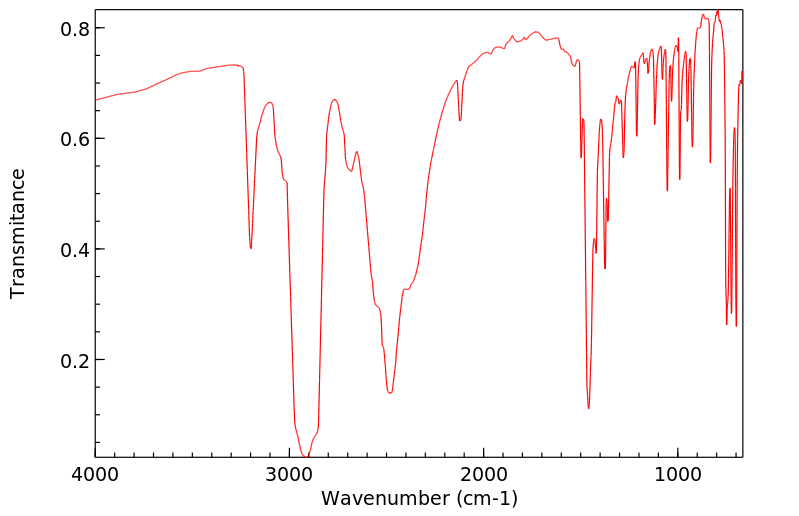
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫