十三烷基磺酸钠 | 5802-89-1
中文名称
十三烷基磺酸钠
中文别名
正十三烷磺酸钠;正十三烷基硫酸钠
英文名称
tridecyl sulfonic acid sodium salt
英文别名
sodium dodecylsulfonate;sodium tridecanesulfonate;tridecane-1-sulfonic acid ; sodium-salt;Tridecan-1-sulfonsaeure; Natrium-Salz;1-Tridecanesulfonic Acid Sodium Salt;sodium;tridecane-1-sulfonate
CAS
5802-89-1
化学式
C13H27O3S*Na
mdl
——
分子量
286.411
InChiKey
CACJZDMMUHMEBN-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
反应信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:185-189°C
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):0.85
-
重原子数:18
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:65.6
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2904100000
文献信息
-
Thermoplastic resin composition and elastomer composition申请人:——公开号:US20040106732A1公开(公告)日:2004-06-03Provided are a thermoplastic resin composition which is excellent in oil resistance, heat resistance, weatherability, impact resistance, transparency, and moldability, and which can be produced economically, an elastomer composition with low hardness and high tensile strength which is excellent in oil resistance and compression set, and a molded object produced by molding the thermoplastic resin composition or elastomer composition. The composition is produced by compounding a thermoplastic resin or an elastomer with a block copolymer having at least one methacrylic ester polymer block and at least one acrylic ester polymer block. Also provided are a process for producing a methacrylic ester-acrylic ester-methacrylic ester block copolymer which requires hardly any purification, which is excellent in heat resistance and weatherability, and in which the molecular weight and the molecular-weight distribution are controlled, and a methacrylic ester-acrylic ester-methacrylic ester block copolymer produced by the process.
-
Process for recovering refractile bodies containing heterologous proteins from microbial hosts and the use thereof申请人:CETUS ONCOLOGY CORPORATION公开号:EP0206828A2公开(公告)日:1986-12-30A refractile material containing a heterologous protein is recovered from a host microorganism cell culture transformed to produce the protein. One recovery process involves disrupting the cell wall and membrane of the host cell, removing greater than 99% by weight of the salts from the disruptate, redisrupting the desalted disruptate, adding a material to the disruptate to create a density or viscosity gradient in the liquid within the disruptate, and separating the refractile material from the cellular debris by high-speed centrifugation (Figure 1 Another version of such a recovery process comprises the further steps of solubilizing the refractile material under reducing conditions, organically extracting the solubilized refractile material, and isolating said refractile material from the extractant (Figure 2). Preferably the protein is recombinant IL-2 or IFN-β and the salt removal step is carried out by diafiltration.
-
Stable formulation of biologically active proteins for parenteral injection申请人:CETUS CORPORATION公开号:EP0217645A2公开(公告)日:1987-04-08A pharmaceutical composition containing IL-2 or IFN-β dissolved in a suitable carrier medium at pH 7.0 to 8.0 stabilized with sodium laurate is suitable for parenteral injection into humans or animals. This formulation may be prepared by adding to either protein, after its recovery from a transformed organism, an effective amount of sodium laurate at a pH of 9 to 9.5 and then adjusting the pH of the formulation to between 7.0 and 8.0. The invention thus provides the use of sodium laurate in stabilizing an interleukin-2 or interferon-β protein for administration to animals or humans.
-
Pharmaceutical compositions of recombinant interleukin-2 and formulation processes申请人:CETUS ONCOLOGY CORPORATION公开号:EP0268110A1公开(公告)日:1988-05-25Stable pharmaceutical compositions suitable for parenteral administration to animals or humans are prepared comprising a therapeutically effective amount of a recombinant interleukin-2 (IL-2) protein dissolved in an inert carrier medium comprising one or more biocompatible non-ionic polymeric detergents which act as solubilizer/stabilizers for the claimed formulations.
-
Pharmaceutical compositions of recombinant beta-interferon and formulation processes申请人:CETUS ONCOLOGY CORPORATION公开号:EP0270799A1公开(公告)日:1988-06-15Stable pharmaceutical compositions suitable for parenteral administration to animals or humans are prepared comprising a therapeutically effective amount of a recombinant IFN-β protein dissolved in an inert carrier medium comprising as solubilizer/stabilizer(s) one or more biocompatible non-ionic polymeric detergents or a combination of one or more biocompatible non-ionic polymeric detergents with an additional solubilizing and/or stabilizing agent, such as sodium dodecyl sulfate or glycerol. Said compositions are in liquid form or lyophilized.
表征谱图
-
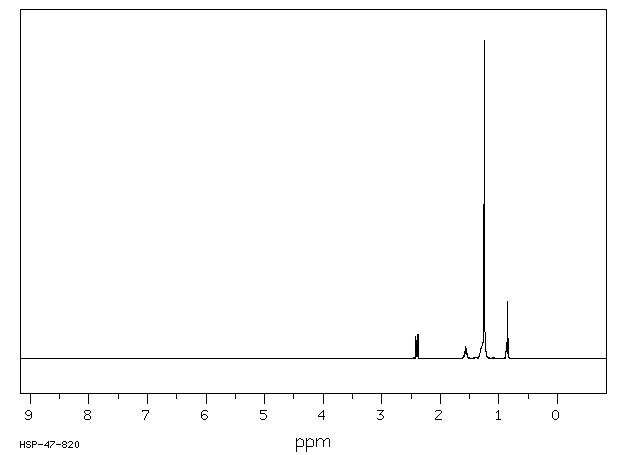
氢谱1HNMR
-
质谱MS
-
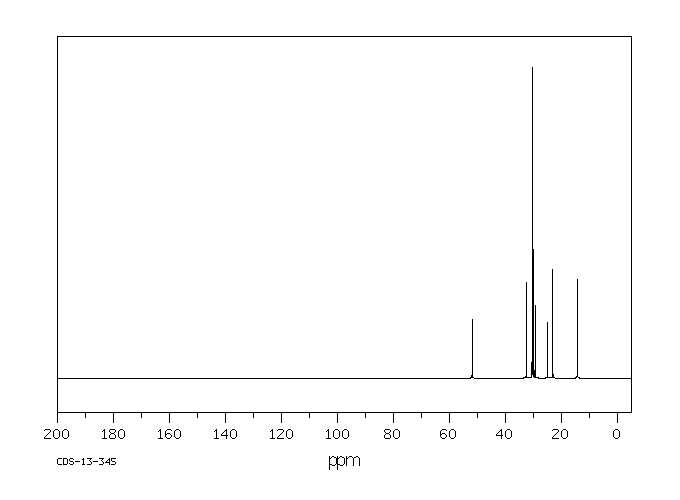
碳谱13CNMR
-
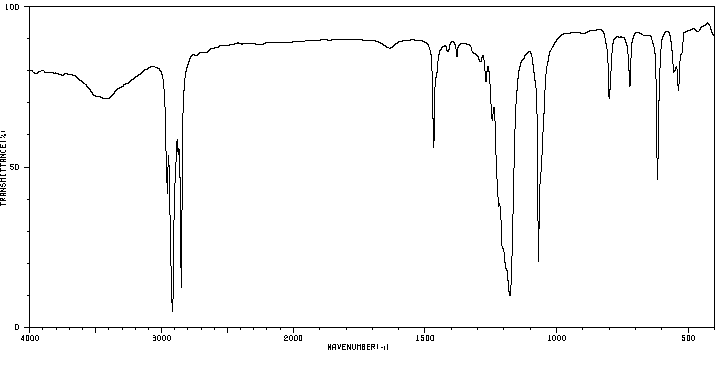
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高烯丙基(甲磺酰基)胺
高炔丙基(甲磺酰基)胺
顺式-9-十八碳烯基甲烷磺酸酯
顺式-4-乙基环己基甲烷磺酸酯
顺-1,2-双(甲磺酰基氧基甲基)环己烷
阿坎酸杂质
阿坎酸
锌甲烷磺酸盐
铵磺酸甜菜碱-3
铵磺酸甜菜碱-2
铵磺酸甜菜碱-1
铬雾抑制剂
铁三(三氟甲基磺酰基)亚胺
钾3-(三羟基硅烷基)-1-丙烷磺酸酯
钾1,1,2,2,3,3,4,4-八氟丁烷-1-磺酸盐
钡二乙烷磺酸酯
钠3-氨基丙烷磺酸酯
钠3-氨基-3-氧代-丙烷-1-磺酸酯
钠3-(三羟基硅烷基)-1-丙烷磺酸酯
钠2-[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-十八碳-9-烯氧基乙氧基)乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]-2-氧代-乙烷磺酸酯
钠1-羟基-1-庚烷磺酸酯
钠(1E)-1-十二碳烯-1-磺酸酯
酪朊酸钠
辛烷-1-磺酸甲酯
辛烷-1-磺酸乙酯
辛基-1-磺酸戊酯
辛-2-烯-1-磺酸
辅酶 M
西尼必利杂质7
萘-1,8-二甲醇
英丙舒凡对甲苯磺酸盐
英丙舒凡
苯基硒基三氟甲烷磺酸酯
芥酸酰胺丙基羟基磺基甜菜碱
艾日布林中间体
脒基牛磺酸
胺磺酸甜菜碱-4
联硫亚盐氯乙醛钠水合物
羧基-五聚乙二醇-磺酸
羟甲基磺酸钠
羟基甲烷磺酸铵盐
羟基甲烷磺酸钾
羟基甲氧基甲醇甲磺酸酯
羟乙磺酸钾
羟乙基磺酸铵盐
羟乙基磺酸钠
羟丙基硫代硫酸钠
美司那
磺酸钠
磺酸己烷