异丁酸异戊酯 | 2445-69-4
中文名称
异丁酸异戊酯
中文别名
异丁酸2-甲基丁酯;2-甲基丁基异丁酸酯; 2-甲基丁基异丁酸酯
英文名称
2-methylpropanoic acid 2-methylbutyl ester
英文别名
2-methylbutyl isobutyric acid ester;2-methylbutyl isobutanoate;2-methylbutyl isobutyrate;isobutyric acid-(2-methyl-butyl ester);Isobuttersaeure-(2-methyl-butylester);2-methyl-propanoic acid, 2-methylbutyl ester;2-methylbutyl 2-methylpropanoate
CAS
2445-69-4
化学式
C9H18O2
mdl
——
分子量
158.241
InChiKey
DUAXUBMIVRZGCO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-73°C (estimate)
-
沸点:183.34°C (estimate)
-
密度:0.8809 (estimate)
-
LogP:3.021 (est)
-
保留指数:989;1001;1001;1002;1004;999;1002;1002;1002;1002;1002;991;1002;1002
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:11
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.89
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915600000
SDS
反应信息
-
作为产物:参考文献:名称:Nematicidal Activity of Natural Ester Compounds and Their Analogues against Pine Wood Nematode, Bursaphelenchus xylophilus摘要:In this study, we evaluated the nematicidal activity of natural ester compounds against the pine wood nematode, Bursaphelenchus xylophilus, to identify candidates for the development of novel, safe nematicides. We also tested the nematicidal activity of synthesized analogues of these ester compounds to determine the structure-activity relationship. Among 28 ester compounds tested, isobutyl 2-methylbutanoate, 3-methylbutyl 2-methylbutanoate, 3-methylbutyl tiglate, 3-methyl-2-butenyl 2-methylbutanoate, and pentyl 2-methylbutanoate showed strong nematicidal activity against the pine wood nematode at a 1 mg/mL concentration. The other ester compounds showed weak nematicidal activity. The LC50 values of 3-methylbutyl tiglate, isobutyl 2-methylbutanoate, 3-methylbutyl 2-methylbutanoate, 3-methyl-2-butenyl 2-methylbutanoate, and pentyl 2-methylbutanoate were 0.0218, 0.0284, 0.0326, 0.0402, and 0.0480 mg/mL, respectively. The ester compounds described herein merit further study as potential nematicides for pine wood nematode control.DOI:10.1021/jf503631e
文献信息
-
[EN] PROCESSES FOR PRODUCING CARBOXYLIC ACIDS<br/>[FR] PROCÉDÉS DE PRODUCTION D'ACIDES CARBOXYLIQUES申请人:EASTMAN CHEM CO公开号:WO2020205348A1公开(公告)日:2020-10-08Processes are disclosed for preparing carboxylic acids from organic esters, the processes comprising contacting an ester with water in the presence of an acid catalyst and a homogenizing solvent at conditions effective to form a carboxylic acid. The homogenizing solvent is present in an amount sufficient to form a single-phase reaction mixture comprising the ester, water, and homogenizing solvent. The homogenizing solvent may be selected from acetonitrile, dimethyl sulfoxide, and 1,4-dioxane.
-
Compositions related to a novel endophytic fungi and methods of use申请人:——公开号:US20040141955A1公开(公告)日:2004-07-22This invention provides pesticidally effective compositions related to a novel endophytic fungi named Muscodor. Specifically, the invention relates to commercially useful formulations of Muscodor and methods for preparing such formulations. It also provides various synthetic pesticidal mixtures of volatile organic compounds isolatable from Muscodor grown on various substrates. Also provided are methods for inhibiting the growth of organisms, such as microbes, insects, and nematodes by exposing such organisms or the habitats thereof to the above Muscodor-related compositions.
-
NEW COMPOUNDS, PHARMACEUTICAL COMPOSITION AND METHODS RELATING THERETO申请人:DYCK Brian公开号:US20110166116A1公开(公告)日:2011-07-07New compounds are disclosed which have utility in the treatment of a variety of metabolic related conditions in a patient. The compounds of this invention have the structure (I): wherein R 1 , R 2 , R 3 , n, p, q, and Ar are as defined herein, including stereoisomers, and pharmaceutically acceptable salts thereof. Also disclosed are pharmaceutical compositions comprising a compound of this invention, as well as methods relating to the use thereof in a patient in need thereof.本发明披露了一种新化合物,其在治疗患者的各种代谢相关疾病方面具有用途。本发明的化合物具有结构(I):其中R1、R2、R3、n、p、q和Ar的定义如本文所述,包括立体异构体和药物可接受的盐。本发明还披露了包括本发明化合物的制药组合物,以及与在需要时使用该化合物的相关方法。
-
Topcoat compositions and pattern-forming methods申请人:Rohm and Haas Electronic Materials LLC公开号:US10042259B2公开(公告)日:2018-08-07Topcoat compositions comprise: a matrix polymer; a surface active polymer comprising a polymerized unit formed from a monomer of the following general formula (I): wherein: R1 represents H, F, methyl or fluorinated methyl; R2 represents optionally substituted C1 to C8 alkylene or optionally substituted C1 to C8 fluoroalkylene, optionally comprising one or more heteroatom; R3 represents H, F, optionally substituted C1 to C10 alkyl or optionally substituted C5 to C15 aryl, optionally comprising one or more heteroatom; R4 represents optionally substituted C1 to C8 alkyl, optionally substituted C1 to C8 fluoroalkyl or optionally substituted C5 to C15 aryl, optionally comprising one or more heteroatom; X represents O, S or NR5, wherein R5 is chosen from hydrogen and optionally substituted C1 to C5 alkyl; and a is 0 or 1; and a solvent. Also provided are coated substrates and pattern-forming methods which make use of the topcoat compositions. The invention has particular applicability in photolithographic processes as a photoresist topcoat layer in the manufacture of semiconductor devices.面漆组合物包括:基质聚合物;表面活性聚合物,该聚合物由以下通式 (I) 的单体聚合而成: 其中R1 代表 H、F、甲基或氟化甲基; R2 代表任选取代的 C1 至 C8 亚烷基或任选取代的 C1 至 C8 氟亚烷基,任选包含一个或多个杂原子; R3 代表 H、F、任选取代的 C1 至 C10 烷基或任选取代的 C5 至 C15 芳基,任选包含一个或多个杂原子;R4 代表任选取代的 C1 至 C8 烷基、任选取代的 C1 至 C8 氟烷基或任选取代的 C5 至 C15 芳基,任选包含一个或多个杂原子;X 代表 O、S 或 NR5,其中 R5 选自氢和任选取代的 C1 至 C5 烷基;a 为 0 或 1;以及溶剂。本发明还提供了涂层基底和图案形成方法,这些基底和方法都使用了本发明的面漆组合物。本发明特别适用于光刻工艺,作为制造半导体器件的光刻胶面涂层。
-
Topcoat compositions containing fluorinated thermal acid generators申请人:Rohm and Haas Electronic Materials LLC公开号:US10241411B2公开(公告)日:2019-03-26Provided are topcoat compositions that include: a matrix polymer; a surface active polymer; an ionic thermal acid generator comprising an anion and a cation, wherein the anion, the cation, or the anion and the cation are fluorinated; and a solvent. Also provided are coated substrates and pattern-forming methods which make use of the topcoat compositions. The invention has particular applicability in photolithographic processes as a photoresist topcoat layer in the manufacture of semiconductor devices.所提供的面漆组合物包括:基质聚合物;表面活性聚合物;由阴离子和阳离子组成的离子热酸发生体,其中阴离子、阳离子或阴离子和阳离子均为氟化;以及溶剂。此外,本发明还提供了涂层基底和图案形成方法,这些基底和方法都使用了本发明的面漆组合物。本发明特别适用于光刻工艺,可用作制造半导体器件的光刻胶面涂层。
表征谱图
-
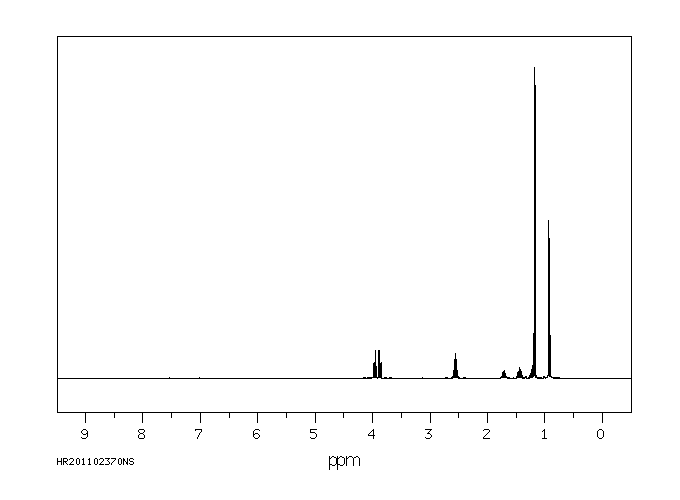
氢谱1HNMR
-
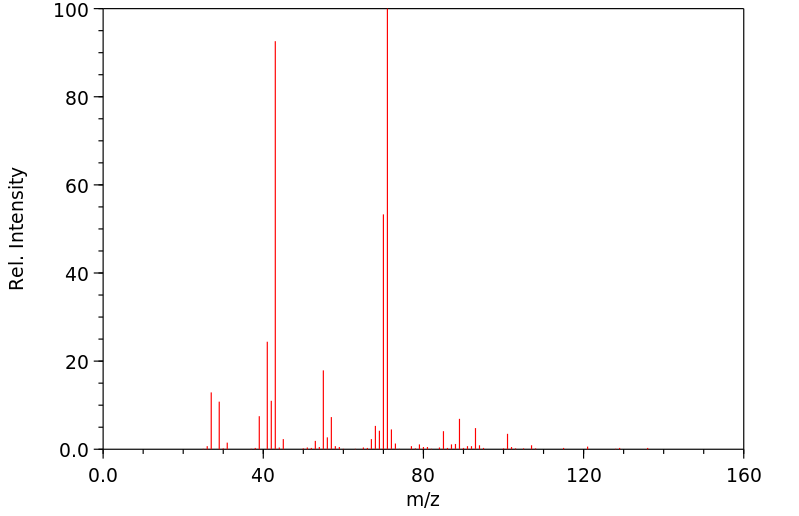
质谱MS
-
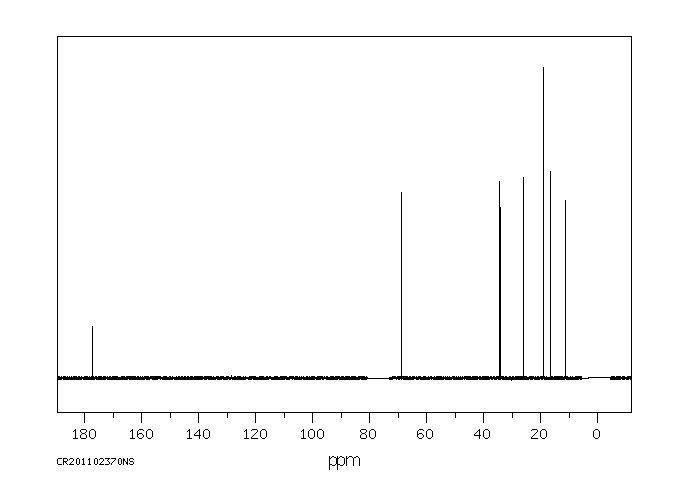
碳谱13CNMR
-
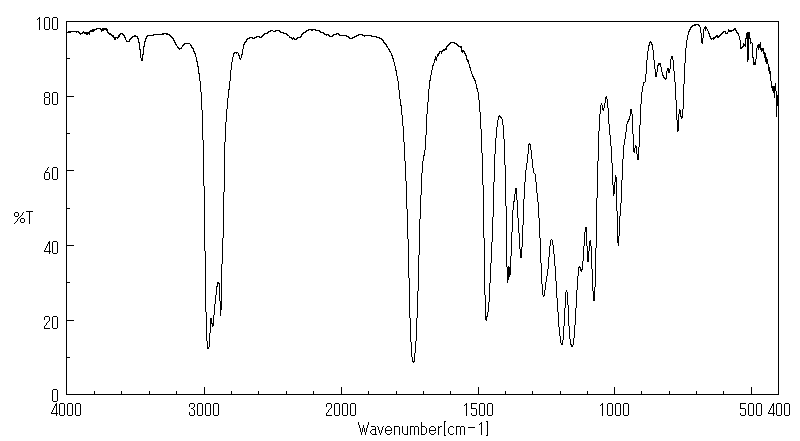
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸