(E)-(3,3-二乙氧基-1-丙烯基)苯 | 25226-98-6
中文名称
(E)-(3,3-二乙氧基-1-丙烯基)苯
中文别名
——
英文名称
trans-cinnamaldehyde diethylacetal
英文别名
(E)-(3,3-diethoxyprop-1-en-1-yl)benzene;Cinnamaldehyde diethyl acetal;[(E)-3,3-diethoxyprop-1-enyl]benzene
CAS
25226-98-6
化学式
C13H18O2
mdl
——
分子量
206.285
InChiKey
VYKDEWVAUWARRX-ZHACJKMWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:120-123 °C(Press: 3 Torr)
-
密度:0.9804 g/cm3
-
保留指数:1445
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:15
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.38
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-苯基丙-2-烯-1-醇 (2E)-3-phenyl-2-propen-1-ol 4407-36-7 C9H10O 134.178 反式-肉桂酸乙酯 ethyl cinnamate 4192-77-2 C11H12O2 176.215
反应信息
-
作为反应物:描述:参考文献:名称:吸附在硅胶上的四氟硼酸作为可重复使用的多相两用催化剂,用于将醛/酮转化为缩醛/缩酮并再次返回摘要:在吸附在硅胶 (HBF4-SiO2) 上的四氟硼酸的催化作用下,用原甲酸三甲酯 (TMOF) 或原甲酸三乙酯 (TEOF) 处理可以将醛和酮保护为缩醛和缩酮。对于具有高度亲电羰基的醛或酮,反应在无溶剂条件下进行。芳基烷基酮、芳基苯乙烯基酮、具有弱亲电羰基的醛和具有可与催化剂配位的取代基的醛需要存在相应的醇作为溶剂。对于可以在纯净条件下转化为缩醛的底物,当使用醇作为溶剂时,缩醛的形成速度更快。催化剂可以回收和再利用/再循环四次(每次使用后重新活化后),其催化效率没有任何显着降低。通过在 HBF4-SiO2 存在下在室温下用水-醇处理短时间从相应的缩醛/缩酮以高产率再生母体醛/酮。在涉及具有不同电子和空间环境的羰基底物的分子间和分子内竞争研究中观察到优异的选择性。苯甲醛的选择性缩醛形成发生在 4-(二甲氨基)苯甲醛、噻吩-2-甲醛、1-萘醛、9-蒽醛或苯乙酮的存在下,但 3-硝基苯甲醛在首选苯甲醛。在DOI:10.1055/s-2008-1042940
-
作为产物:描述:参考文献:名称:Dombrowskii; Jurkewitsch; Terent'ew, Zhurnal Obshchei Khimii, 1957, vol. 27, p. 3047,3049摘要:DOI:
文献信息
-
Sulfamic Acid as a Cost‐Effective and Recyclable Catalyst for Protection of Carbonyls to Acetals and Ketals Under Mild Conditions作者:Weizhong Gong、Bo Wang、Yanglong Gu、Liang Yan、Liming Yang、Jishuan SuoDOI:10.1081/scc-200039314日期:2004.12.31Abstract An efficient H2NSO3H‐catalyzed protection of various carbonyl compounds at room temperature was investigated. The features of mild conditions, cost‐efficient catalyst, simple workup, and the recyclability of the catalyst were represented in this process.摘要 研究了在室温下 H2NSO3H 催化的各种羰基化合物的有效保护。该工艺具有条件温和、催化剂成本低、后处理简单、催化剂可回收等特点。
-
Asymmetric simmons-smith reactions using homochiral protecting groups作者:Atsunori Mori、Isao Arai、Hisashi Yamamoto、Hisao Nakai、Yoshinobu AraiDOI:10.1016/s0040-4020(01)88107-2日期:1986.1Asymmetric Simmons-Smith reactions of α,β-unsaturated acetals derived from chiral dialkyl tartrate or (2R,4R)-2,4-pentanediol are described. Treatment of the acetal with diethylzinc and methylene iodide gives a cyclopropane with high diastereoselectivity. The acetal group is readily transformed to the aldehyde or the ester group. In addition, the method is successfully applied to the enantioselective
-
2,3-Dichloro-5,6-dicyano-<i>p</i>-benzoquinone (DDQ) as a Highly Efficient and Mild Catalyst for Diethyl Acetalization of Carbonyl Compounds作者:Babak Karimi、Ashraf Miri AshtianiDOI:10.1246/cl.1999.1199日期:1999.11Various types of structurally different carbonyl compounds in the presence of ethyl orthoformate (EtO)3CH could be efficiently converted to their diethyl acetals by using a catalytic amount (1-3 mol%) of DDQ under mild reaction conditions.
-
An Efficient Palladium-Catalyzed Synthesis of Cinnamaldehydes from Acrolein Diethyl Acetal and Aryl Iodides and Bromides作者:Gianfranco Battistuzzi、Sandro Cacchi、Giancarlo FabriziDOI:10.1021/ol034071p日期:2003.3.1The reaction of aryl iodides and bromides with acrolein diethyl acetal in the presence of Pd(OAc)(2), (n)()Bu(4)NOAc, K(2)CO(3), KCl, and DMF, at 90 degrees C until the disappearance of the acetal followed by the addition of 2 N HCl to the crude reaction mixture, affords cinnamaldehydes in good to high yields. A variety of functional groups are tolerated in the aryl halides, including ether, aldehyde
-
An Efficient and Versatile Procedure for the Synthesis of Acetals from Aldehydes and Ketones Catalyzed by Lithium Tetrafluoroborate作者:Tsuneo Sato、Nao Hamada、Kiyoshi Kazahaya、Hisashi ShimizuDOI:10.1055/s-2004-820038日期:——Acetals are obtained in good to excellent yields by treatment of aldehydes and ketones with trialkyl orthoformate and the corresponding alcohol in the presence of a catalytic amount of lithium tetrafluoroborate. Due to the mild reaction conditions, this method is compatible with acid-sensitive substrates.
表征谱图
-
氢谱1HNMR
-
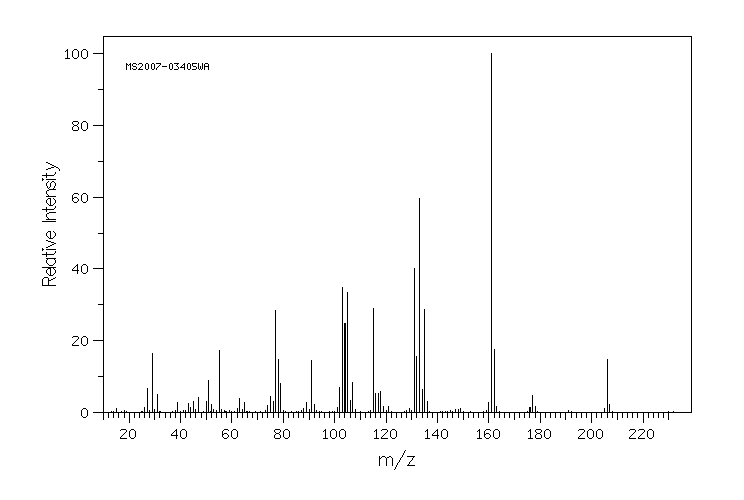
质谱MS
-
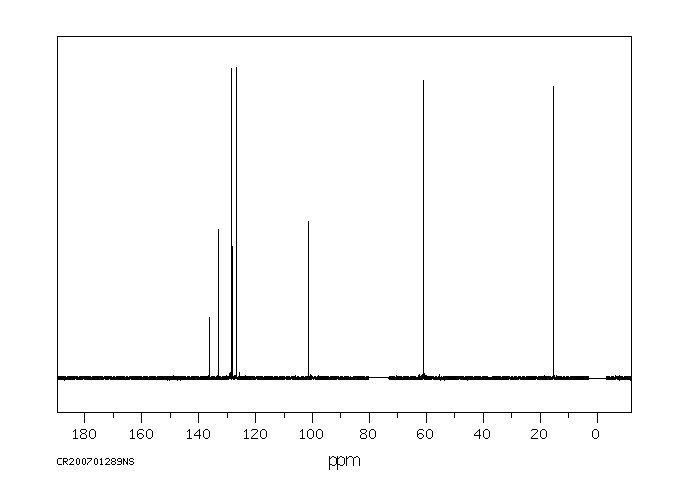
碳谱13CNMR
-
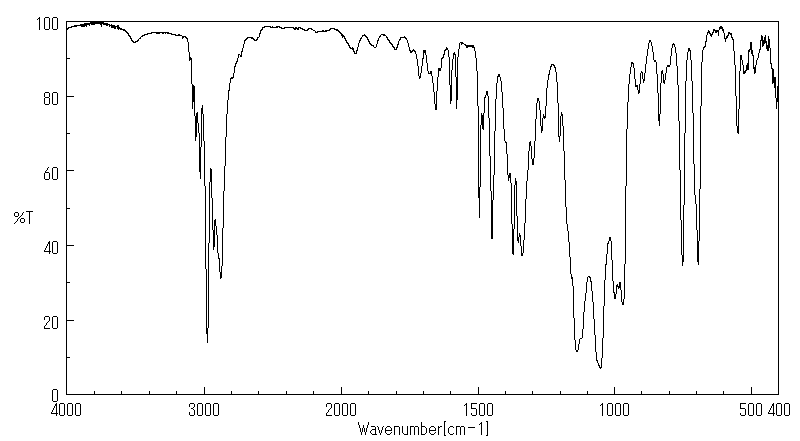
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫