(三氯甲基)环氧乙烷 | 3083-23-6
中文名称
(三氯甲基)环氧乙烷
中文别名
——
英文名称
1,1,1-trichloro-2,3-epoxypropane
英文别名
Trichloroepoxypropane;3,3,3-trichloropropylene oxide;1,2-epoxy-3,3,3-trichloropropane;2-(trichloromethyl)oxirane;trichloromethyl-oxirane
CAS
3083-23-6
化学式
C3H3Cl3O
mdl
MFCD00055951
分子量
161.415
InChiKey
VFEXYZINKMLLAK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:226.05°C (rough estimate)
-
密度:1.4950
-
物理描述:1,2-epoxy-3,3,3-trichloropropane is a clear colorless liquid. (NTP, 1992)
-
闪点:152 °F (NTP, 1992)
-
溶解度:less than 0.1 mg/mL at 65.3° F (NTP, 1992)
-
蒸汽压力:11.6 mm Hg at 77 °F ; 28.5 mm Hg at 108° F; 65.5 mm Hg at 149° F (NTP, 1992)
-
保留指数:885
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12.5
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (+/-)-3,3,3-Trichlor-propan-1,2-diol 815-02-1 C3H5Cl3O2 179.43
反应信息
-
作为反应物:描述:参考文献:名称:Halogen-Metal Interchange Reactions of 3,3,3-Trichloro-1,2-epoxypropane and of Chloral with Organolithium Compounds and Grignard Reagents摘要:DOI:10.1021/ja01059a027
-
作为产物:描述:参考文献:名称:Meerwein; Bersin; Burneleit, Chemische Berichte, 1929, vol. 62, p. 1006摘要:DOI:
-
作为试剂:描述:2-(1-甲基乙烯)环氧乙烷 在 (三氯甲基)环氧乙烷 、 NADPH-generating system 、 rat liver microsomal proteins 作用下, 以 phosphate buffer 为溶剂, 反应 1.0h, 生成 (2R,2'S)-2-methyl-2,2'-bioxirane 、 (2S,2'S)-2-methyl-2,2'-bioxirane 、 (2S,2'R)-2-methyl-2,2'-bioxirane 、 (2R,2'R)-2-methyl-2,2'-bioxirane参考文献:名称:异戊二烯单环氧化物和相应二醇与对照和诱导大鼠肝脏微粒体的生物转化的立体化学过程。摘要:已经确定了来自对照和诱导大鼠的肝酶对异戊二烯进行生物转化的立体化学过程。在两个主要形成的代谢物2-甲基-2-乙烯基环氧乙烷(2)和异丙烯基环氧乙烷(3)之间,环氧化物2主要通过非酶水解反应迅速转变为相应的邻位外消旋二醇4。在不同的情况下,环氧化物3主要通过微粒体环氧化物水解酶(mEH)生物转化为二醇5,以在50%转化率之前选择性地生成(R)-3-甲基-3-丁烯-1,2-二醇5。与单环氧化物3氧化成相应的二环氧化物6竞争。P450催化的3的环氧化的特征在于中等的立体选择性,然而,立体选择性强烈地依赖于P450的诱导。用苯巴比妥(PB)(P450 2B1和3A的诱导剂)治疗大鼠可产生高选择性苏-(2R,2'R)-6,而用吡唑(Pyr)(P450 2E1的诱导剂)处理,有利于同时形成赤型-(2S,2'R)-和苏型-(2R,2'R)-6。mEH催化的双环氧化物6的水解进行,尽管具有适度的周转率,但DOI:10.1021/tx000061a
文献信息
-
Boron atom reactions. Rate constants with the epoxides作者:R. Estes、M.B. Tabacco、T.G. Digiuseppe、P. DavidovitsDOI:10.1016/0009-2614(82)80177-2日期:1982.3The reaction rates of atomic boron with various epoxides have been measured in a flow tube apparatus. The bimolecular rate constants, in units of cm3 molecule−1 s−1, are: 1,2-epoxypropane (8.6 × 10−11 ), 1,2-epoxybutane (8.8 × 10−11), 1,2,3,4-diepoxybutane (5.5 × 10−11), 1-chloro-2,3-epoxypropane (5.7 × 10−11), and 1,2-epoxy-3,3,3-trichloropropane (1.5 × 10−11).
-
5-Halopyrimid-2-ones申请人:Nyegaard & Co. A/S公开号:US04395406A1公开(公告)日:1983-07-26Novel derivatives of pyrimid-2-one having interesting pharmacological properties are described. The compounds of the invention have been found to be of use in the control of, and in particular in the inhibition of the metaphase of malignant tumours and leukaemias. Processes for the preparation of the novel compounds and pharmaceutical compositions containing them are also described.
-
Reactions of group V organometalloidal compounds. Part VI. Ring opening of β-propiolactone and of epoxides with dialkylamino(dimethyl)-arsines作者:Jugo Koketsu、Yoshio IshiiDOI:10.1039/j39710000002日期:——Treatment of β-propiolactone with dialkylamino(dimethyl)arsines at 80° in benzene gave dimethylarsenic 3-dialkylaminopropionates, formed by alkyl–oxygen bond fission. The addition reactions of dialkylamino(dimethyl)arsines with 1,1,1-trichloro-2,3-epoxypropane took place with normal fission of the epoxide ring to give 2-dialkylamino-1-trichloromethylethoxy(dimethyl)arsines. Although 1-chloro-2,3-epoxypropane
-
Trihalogenomethyl compounds of potential therapeutic interest. Part VIII. Synthesis of some epoxides, amides, alcohols, and acetylenic amines作者:R. E. Bowman、K. D. Brunt、C. E. Harrison、W. R. N. WilliamsonDOI:10.1039/j39700000094日期:——Syntheses of epoxides and amides of the general structure Cl3[graphic omitted] their hydroxy-derivatives, some acetylenic trichloromethanols, and Mannich derivatives of acetylenic trichloromethanols are described.
-
Absence of the benzylic effect in nucleophilic ring opening of oxiranes作者:Jack I. Lynas-Gray、Charles J. M. StirlingDOI:10.1039/c39840000483日期:——In reactions of oxiranes with sulphur nucleophiles under conditions in which kobs. is pH independent, aryloxiranes show none of the expected enhancement of reactivity; conformational restriction of orthogonal substitution accounts for the observations.
表征谱图
-
氢谱1HNMR
-
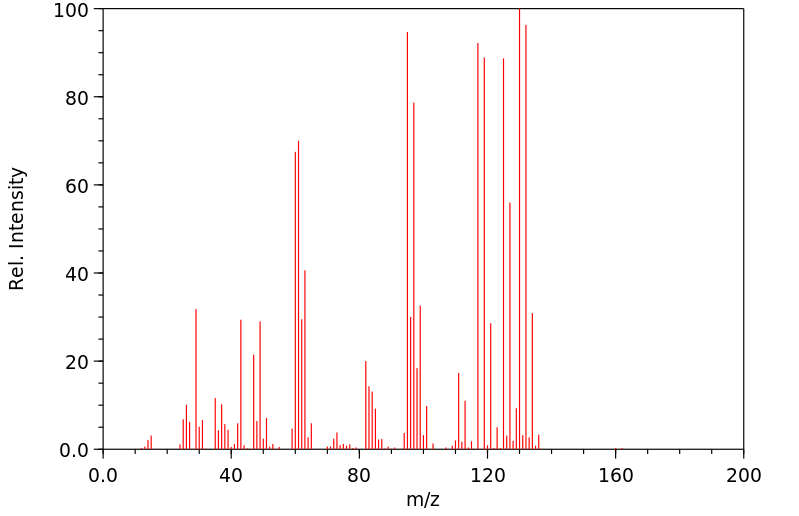
质谱MS
-
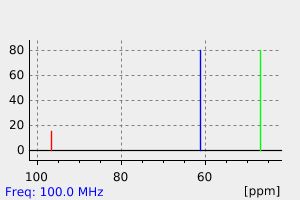
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-氯-1,2-环氧丁烷
顺式-环氧琥珀酸氢钾
顺式-1-环己基-2-乙烯基环氧乙烷
顺-(2S,3S)甲基环氧肉桂酸酯
雌舞毒蛾引诱剂
阿洛司他丁
辛基缩水甘油醚
试剂(3S,6S)-(-)-3,6-Diisopropyl-1,4-dioxane-2,5-dione
表氰醇
螺[环氧乙烷-2,2-三环[3.3.1.1~3,7~]癸烷]
蛇根混合碱
benzene oxide
聚碳酸丙烯酯
聚依他丁
羟基乙醛
缩水甘油基异丁基醚
缩水甘油基十六烷基醚
缩水甘油
硬脂基醇聚氧乙烯聚氧丙烯醚
硅烷,三甲基[(3-甲基噁丙环基)乙炔基]-,顺-
盐酸司维拉姆
甲醛与(氯甲基)环氧乙烷,4,4-(1-甲基乙亚基)双酚和2-甲基苯酚的聚合物
甲醛与(氯甲基)环氧乙烷,4,4'-(1-甲基乙亚基)二[苯酚]和4-(1,1,3,3-四甲基丁基)苯酚的聚合物
甲醇环氧乙烷与壬基酚的聚合物
甲胺聚合物与(氯甲基)环氧乙烷
甲硫代环氧丙烷
甲基环氧氯丙烷
甲基环氧巴豆酸酯
甲基环氧乙烷与环氧乙烷和十六烷基或十八烷基醚的聚合物
甲基环氧乙烷与[(2-丙烯基氧基)甲基]环氧乙烷聚合物
甲基环氧丙醇
甲基环氧丙烷
甲基N-丁-3-烯酰甘氨酸酸酯
甲基7-氧杂双环[4.1.0]庚-2,4-二烯-1-羧酸酯
甲基3-环丙基-2-环氧乙烷羧酸酯
甲基1-氧杂螺[2.5]辛烷-2-羧酸酯
甲基(2S,3R)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3S)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3R)-3-环丙基-2-环氧乙烷羧酸酯
环氧溴丙烷
环氧氯丙烷与双酚A、4-(1,1-二甲乙基)苯酚的聚合物
环氧氯丙烷-d5
环氧氯丙烷-D1
环氧氯丙烷-3,3’-亚氨基二丙胺的聚合物
环氧氯丙烷-2-13C
环氧氯丙烷
环氧氟丙烷
环氧树脂(环氧氯丙烷和二乙二醇)
环氧树脂
环氧柏木烷