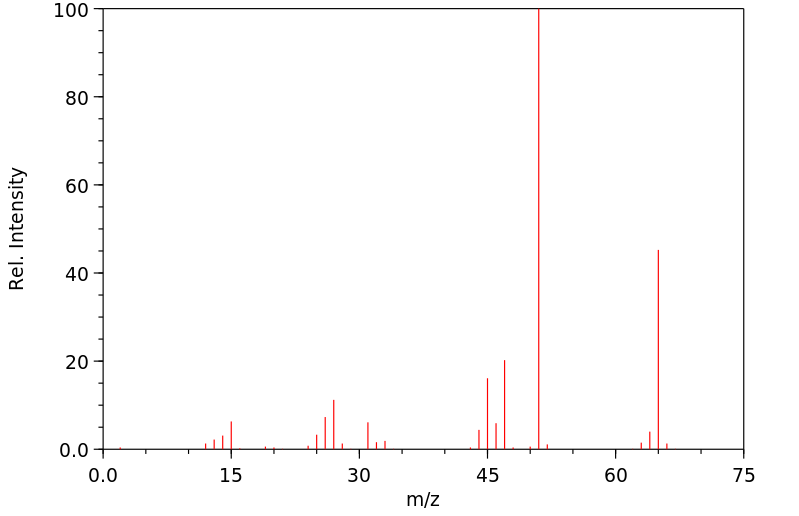
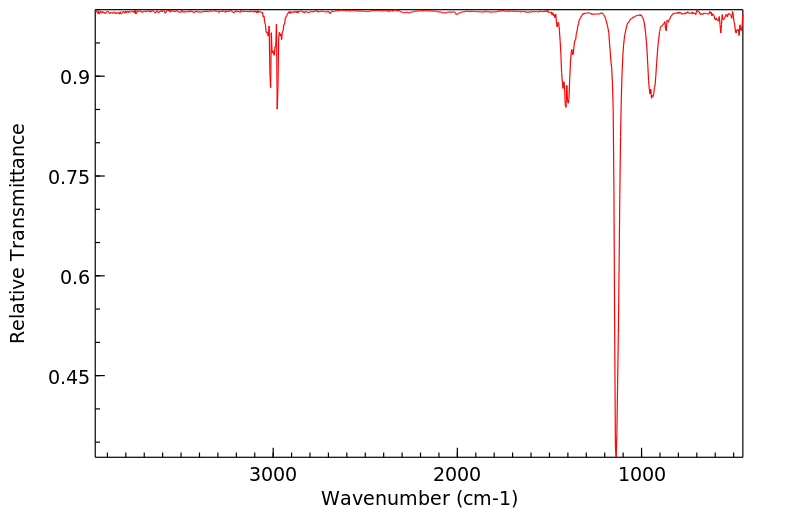
毒理性
识别和使用:1,1-二氟乙烷(HFC-152a)是一种无色、无味的气体。HFC-152a的主要用途是作为气溶胶推进剂和泡沫膨胀剂。其他潜在用途包括制冷混合物和催化剂再生。是制备乙烯氟化物的中间体。人类暴露和毒性:几名志愿者暴露于500,000 ppm的HFC 152a几分钟后,报告出现了镇痛和即将失去意识的情况。液体的快速蒸发可能导致冻伤。该物质可能对心血管系统产生影响,导致心脏疾病。氟化烃的滥用正在上升,特别是在青少年人群中。吸入由HFC-152a组成的气溶胶电脑清洁喷雾导致了明显的上下唇面部肿胀,与血管性水肿相符。患者还出现了QT间期延长、轻度吸气性喉鸣,但没有荨麻疹。在另一个案例中,连续两天几乎不间断地吸食后,出现了急性心肌损伤、全心力减弱、横纹肌溶解、急性肾损伤和暴发性肝炎。卤代烃吸入滥用的一种严重但很少报告的并发症是严重的粘膜冻伤。在体外人类淋巴细胞染色体畸变试验中,HFC-152a显示出微弱的致裂变性。
动物研究:HFC-152a具有较低的急性吸入毒性。重复剂量研究表明有一定的刺激性。HFC-152a可能在狗同时接触高浓度暴露和高剂量外源性肾上腺素时产生心脏过敏。在为期2周的重复剂量吸入研究中,大鼠在100,000 ppm的暴露水平下,HFC-152a显示出麻醉性质。在大鼠吸入25,000 ppm HFC-152a三个月的暴露后,没有观察到不良反应。在一项发育研究中,雌性大鼠在怀孕的第6天至第15天,每天6小时,通过吸入暴露至50,000 ppm。在任何测试的浓度下都没有观察到与化合物相关的母体和发育影响。在雄性和雌性大鼠每天6小时,每周5天,暴露于25,000 ppm HFC-152a 3、12或24个月的实验中,没有观察到生殖器官的的组织病理学或重量影响。在一项为期2年的生物鉴定中,HFC-152a在大鼠吸入暴露水平高达25,000 ppm时,不具有致癌性。HFC-152a在Salmonella typhimurium和Escherichia coli菌株的体外细菌反向突变试验(Ames试验)中不具有致突变性。在大鼠体内微粒体试验中,通过全身吸入给药,没有显示出染色体损伤或骨髓细胞毒性的证据。
IDENTIFICATION AND USE: 1,1-Difluoroethane (HFC-152a) is a colorless, odorless gas. The primary uses for HFC-152a are as an aerosol propellant and a foam expansion agent. Other potential uses include refrigeration blends and catalyst regeneration. Intermediate to vinyl fluoride. HUMAN EXPOSURE AND TOXICITY: Several volunteers were exposed to 500,000 ppm of HFC 152a for several min. Analgesia and an impending loss of consciousness were reported. Rapid evaporation of the liquid may cause frostbite. The substance may cause effects on the cardiovascular system , resulting in cardiac disorders. Abuse of fluorinated hydrocarbons is on the rise, especially among the adolescent population. Inhaling aerosolized computer-cleaning spray composed of HFC-152a led to marked upper and lower lip facial swelling consistent with angioedema. The patient also had a prolonged QT interval, mild inspiratory stridor, but no urticaria. In other case, acute myocardial injury and global hypokinesis along with rhabdomyolysis, acute kidney injury, and fulminant hepatitis developed after 2 days of nearly continuous huffing. A serious but rarely reported complication of halogenated hydrocarbon inhalation abuse is severe mucosal frostbite. HFC-152a showed evidence of weak clastogenicity in an in vitro human lymphocyte chromosome aberration test. ANIMAL STUDIES: HFC-152a has low acute inhalation toxicity. The repeat dose studies show some potential for irritation. HFC-152a has the potential to produce cardiac sensitization in dogs challenged simultaneously with high exposure concentrations and high doses of exogenous epinephrine. HFC-152a had anesthetic properties at a 100,000 ppm exposure level during a 2-week repeated dose inhalation study in rats. No adverse effects were observed in rats following a 3-month inhalation exposure to 25,000 ppm HFC-152a. In a developmental study, female rats were exposed via inhalation up to 50,000 ppm during days 6 to 15 of pregnancy for 6 hours per day. No compound related maternal and developmental effects were observed at any of the concentrations tested. No histopathological or weight effects on reproductive organs were observed in male and female rats exposed up to 25,000 ppm HFC-152a for 6 hours per day, 5 days per week for 3, 12 or 24 months. . In a 2-year bioassay, HFC-152a was not carcinogenic to rats at inhalation exposure levels up to 25,000 ppm. HFC-152a was not mutagenic in the in vitro bacterial reverse mutation test (Ames test) in Salmonella typhimurium and Escherichia coli strains. An in vivo rat Micronucleus Test did not show any evidence of chromosome damage or bone marrow cell toxicity when administered by whole body inhalation.
来源:Hazardous Substances Data Bank (HSDB)