1,2-双(6-甲基吡啶-2-基)-1,2-乙二酮 | 6630-11-1
中文名称
1,2-双(6-甲基吡啶-2-基)-1,2-乙二酮
中文别名
——
英文名称
1,2-bis(6-methylpyridin-2-yl)ethane-1,2-dione
英文别名
6,6'-dimethyl-2,2'-pyridil;6.6'-Dimethyl-α-pyridil;bis-(6-methyl-pyridin-2-yl)-ethanedione;bis-(6-methyl-[2]pyridyl)-ethanedione;Bis-(6-methyl-[2]pyridyl)-aethandion;bis(6-methyl-2-pyridyl) diketone
CAS
6630-11-1
化学式
C14H12N2O2
mdl
——
分子量
240.261
InChiKey
GXRQKSBITVLBQD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:59.9
-
氢给体数:0
-
氢受体数:4
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 羟基(6-甲基-2-吡啶基)甲基6-甲基-2-吡啶基甲酮 2-hydroxy-[1,2-bis(6-methylpyridin-2-yl)]ethan-1-one 7252-50-8 C14H14N2O2 242.277 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 羟基(6-甲基-2-吡啶基)甲基6-甲基-2-吡啶基甲酮 2-hydroxy-[1,2-bis(6-methylpyridin-2-yl)]ethan-1-one 7252-50-8 C14H14N2O2 242.277
反应信息
-
作为反应物:描述:1,2-双(6-甲基吡啶-2-基)-1,2-乙二酮 在 lead(II) oxide 作用下, 生成 bis(6-methylpyridin-2-yl)methanone参考文献:名称:Mathes; Sauermilch, Chemische Berichte, 1953, vol. 86, p. 109摘要:DOI:
-
作为产物:描述:参考文献:名称:2,2′-Pyridoin derivatives protect HL-60 cells against oxidative stress摘要:Focusing on 2,2 '-pyridoin (1, 1,2-di(2-pyridyl)-1,2-ethenediol) and its synthetic derivatives as the lead compound of the potent antioxidative enediol, their protective effect against oxidative stress was evaluated on the HL-60 cell system. 2,2 '-Pyridoins showed no remarkable cytotoxic effect on HL-60 cells. The derivatives 1, 2, 3, 5, and 6 inhibited H(2)O(2)-induced cell death and intracellular oxidative stress more significantly than ascorbic acid. Since 2,2 '-pyridoins are oxidized to the diketones, 2,2 '-pyridils, in a protic solvent, the antioxidant activity of 2,2 '-pyridils was also investigated. 2,2 '-Pyridils showed antioxidant activity in the cell; however, the activity was lower than that of 2,2 '-pyridoins. These results suggested that 2,2 '-pyrdoin derivatives can be good cytoprotective agents against oxidative stress. (c) 2008 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2008.08.057
文献信息
-
Metal-to-ligand charge-transfer excited-states in binuclear copper(I) complexes. Tuning MLCT excited-states through structural modification of bridging ligands. A resonance Raman study作者:Mark R. Waterland、Amar Flood、Keith C. GordonDOI:10.1039/a909121a日期:——The resonance Raman spectra of the ground- and lowest excited-states for a series of binuclear copper(I) complexes with bridging ligands based on 2,3-di-(2-pyridyl)quinoxaline have been measured. Analyses of the ground-state resonance Raman spectra show strong enhancement of an inter-ring stretching mode and quinoxaline-based modes; consistent with the Frank–Condon state having bonding changes about
-
Synthesis, Characterisation and Crystal Structure of a Co-crystal of Two Components: 1,2-bis(6-methylpyridin-2-yl)ethane-1,2-dione and 1-(pyridin-2-yl)-2-(6-methylpyridin-2-yl)ethane-1,2-dione作者:M. Judith Percino、Víctor M. Chapela、Omar Urzúa、Héctor Toribio、Cecilia Rodríguez-BarbarínDOI:10.3184/030823407x200434日期:2007.3A condensation reaction of an equimolar ratio of 2-pyridinecarboxaldehyde and (6-methylpyridin-2-yl)methanol without a catalyst in a solvent-free reaction at 140°C was expected to yield 2-hydroxy-[1,2-bis(6-methylpyridin-2-yl)]ethan-1-one. FT-IR spectroscopy of the yellow-brown solid reaction product indicated the presence of a –CO–COH– group. The solid compound was treated with cyclohexane and green2-吡啶甲醛和(6-甲基吡啶-2-基)甲醇等摩尔比的缩合反应在无催化剂的情况下,在140°C下的无溶剂反应中预计会产生2-羟基-[1,2-双( 6-methylpyridin-2-yl)]ethan-1-one。黄褐色固体反应产物的 FT-IR 光谱表明存在 -CO-COH- 基团。固体化合物用环己烷处理,产生绿色晶体。通过FT-IR、1H NMR、EI质谱和单晶X射线衍射对晶体进行表征。通过单晶 X 射线衍射确定的分子和晶体结构解析出一种晶体,该晶体显示两种分子组分 [C13H10N2O2 和 C14H12N2O2],比例为 60.4:39.6 [C13.40H10.79N2O2]。两种化合物在三斜空间群 P-1 中共结晶,a = 7.859(5), b = 8.021(14), c = 9.060(5) Å,α = 101.36(2)°, β = 90.06(3)°, γ = 90.92(3)°
-
Process to obtain dimers, trimers and up to polymers from pyridinmethanol derivatives compounds申请人:PERCINO ZACARIAS Maria Judith公开号:US20090043063A1公开(公告)日:2009-02-12The process of the current invention, named Percino-Chapela, has as one of its main novelty features that starting from pyridinmethanol derivatives, a dimerization or polymerization reaction of pyridinic alcohols is carried out in order to produce novel products, the process of the current invention has the following aspects that characterize it: it is carried out in the absence or presence of some solvent, during the process of the current invention, temperature may be or may be not used as catalyst, in the process of the current invention the reaction may be or may not be catalyzed by the presence of a catalyst (acid or base), the resultant products can be produced and separated in an easy way, in the process of the current invention starting from pyridinic alcohols the resultant ethenediols can be produced by a single step reaction, the pyridinmethanol derivatives used as starting compounds, do not oxidize as easily and their handling is easier than that of other compounds previously used that oxidize easily, the products produced with etheneidol parts can be used as antioxidants due to their capacity to act as free radicals scavengers.
-
Klosa, Journal fur praktische Chemie (Leipzig 1954), 1959, vol. <4> 9, p. 289作者:KlosaDOI:——日期:——
-
�ber eine der Benzils�ureumlagerung analoge Reaktion in der Pyridinreihe作者:Josef KlosaDOI:10.1007/bf00641072日期:——
表征谱图
-
氢谱1HNMR
-
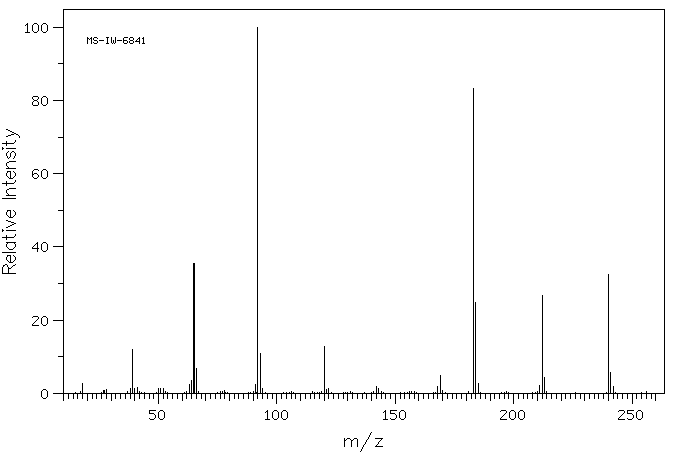
质谱MS
-
碳谱13CNMR
-
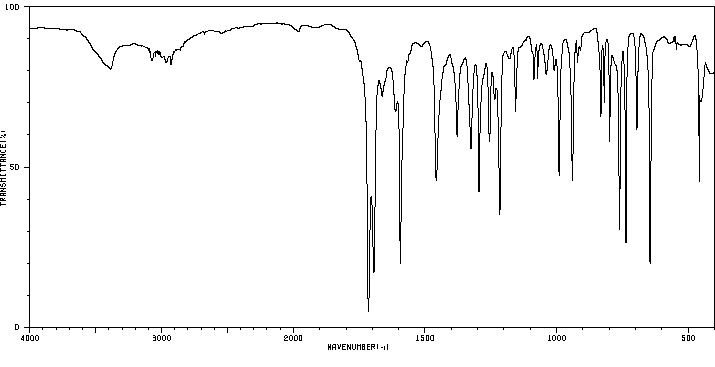
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷