1,3-二溴-1,3-二苯基丙烷-2-酮 | 958-79-2
分子结构分类
中文名称
1,3-二溴-1,3-二苯基丙烷-2-酮
中文别名
——
英文名称
1,3-dibromo-1,3-diphenylpropan-2-one
英文别名
α,α'-dibromodibenzyl ketone;1,3-dibromo-1,3-diphenyl-propan-2-one;1,3-diphenyl-1,3-dibromopropa-3-one;1,3-dibromo-1,3-diphenylacetone;1,3-dibromo-1,3-diphenyl-acetone;1,3-Dibrom-1,3-diphenyl-aceton;1,3-Dibromo-1,3-diphenyl-2-propanone
CAS
958-79-2
化学式
C15H12Br2O
mdl
MFCD00013537
分子量
368.068
InChiKey
AKVJDXBLRZFJFZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:112-114 °C
-
沸点:399.5±37.0 °C(Predicted)
-
密度:1.639±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:18
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.13
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二苄基甲酮 1,3-Diphenylacetone 102-04-5 C15H14O 210.276
反应信息
-
作为反应物:描述:参考文献:名称:有机催化的[3 + 2]环丙烯酮和β-酮酸酯的环化:一种用季中心取代丁烯内酯的方法摘要:已经开发出前所未有的有机催化的环戊烯酮和β-酮酸酯的[3 + 2]环。该反应提供了直接的方法,以中等到良好的产率制备具有季中心的高度取代的丁烯内酯。初步机理研究证实,烯醇中间体对反应结果至关重要,并且涉及分子间酯化和分子内迈克尔加成过程。DOI:10.1021/acs.orglett.6b03737
-
作为产物:参考文献:名称:醇的芳族阳离子活化:使用二氯二苯基环丙烯转化为烷基氯化物摘要:描述了通过形成环丙烯醚将醇活化为亲核置换的新范例。一系列醇底物在用 3,3-二氯-1,2-二苯基环丙烯处理后迅速转化为相应的烷基氯。(1) H NMR 数据支持环丙烯中间体的中间体,并且证明该反应主要通过 1-苯基乙醇的 S(N)2 机制进行。总共提供了 12 个基板范围的示例。DOI:10.1021/ja906520p
文献信息
-
Iron‐Catalyzed One‐Step Synthesis of Isothiazolone/1,2‐Selenazolone Derivatives via [3+1+1] Annulation of Cyclopropenones, Anilines, and Elemental Chalcogens作者:Hongchen Wang、Rulong YanDOI:10.1002/adsc.202101175日期:2022.2.15Described herein is the one-step synthesis of isothiazolone/1,2-selenazolone derivatives via [3+1+1] cycloaddition of cyclopropenone derivatives, anilines, and elemental chalcogens. The cascade reaction involves the C−S, C−N, and N−S bond formation along with the cleavage of C−C bond. Both anilines and cyclopropenones are tolerated and give the corresponding products in 28–73% yields.
-
Asymmetric Intramolecular Desymmetrization of <i>meso</i>-α,α′-Diazido Alcohols with Aryldiazoacetates: Assembly of Chiral C<sub>3</sub> Fragments with Three Continuous Stereocenters作者:Jin-Bao Qiao、Yu-Ming Zhao、Peiming GuDOI:10.1021/acs.orglett.6b00570日期:2016.5.6The chiral Cu-complex-catalyzed intramolecular interception of meso-α,α′-diazido alcohols with aryldiazoacetates is explored. Most of the enantioenriched α-imino esters with three continuous stereocenters are produced with good to excellent yield and enantioselectivity, and a chiral pocket model is proposed for rationalization of the asymmetric desymmetrization.
-
Organocatalytic Regiodivergent C−C Bond Cleavage of Cyclopropenones: A Highly Efficient Cascade Approach to Enantiopure Heterocyclic Frameworks作者:Jian Cao、Ran Fang、Jin‐Yu Liu、Hong Lu、Yong‐Chun Luo、Peng‐Fei XuDOI:10.1002/chem.201803861日期:2018.12.17reported that combines a cycloaddition reaction with a regioselective strain‐release process to afford diverse heterocyclic frameworks through bifunctional catalysis. The cooperation of hydrogen‐bonding network activation and a regiodivergent strain‐assisted effect is the key to promoting this complex chemical transformation, leading to the generation of two different ring systems in high yields with excellent此处报道了一种高效的级联方法,该方法将环加成反应与区域选择性菌株释放过程结合在一起,通过双功能催化作用提供了多种杂环骨架。氢键网络激活和区域发散应变辅助效应的合作是促进这种复杂化学转化的关键,从而导致以高产率生成具有优良立体选择性的两种不同的环系统。反应是通过一种机制进行的,该机制涉及“弹簧加载”的中间体,该中间体通过可控的环应变释放而实现可切换的C-C键断裂。该反应也适用于仅以0.1摩尔%的催化剂负载量进行克规模的合成。
-
Fully Substituted Conjugate Benzofuran Core: Multiyne Cascade Coupling and Oxidation of Cyclopropenone作者:Liangliang Yao、Qiong Hu、Li Bao、Wenjing Zhu、Yimin HuDOI:10.1021/acs.orglett.1c01304日期:2021.7.2multiyne cascade coupling has been developed. This chemistry provides a novel, simple, and efficient approach to synthesize fully substituted conjugate benzofuran derivatives from simple substrates under mild conditions. The density functional theory (DFT) calculations reveal that the unique homolytic cleavages of cyclopropenone and molecular oxygen are crucial to the success of this reaction.
-
Cyclopropenone Catalyzed Substitution of Alcohols with Mesylate Ion作者:Eric D. Nacsa、Tristan H. LambertDOI:10.1021/ol302970c日期:2013.1.4The cyclopropenone catalyzed nucleophilic substitution of alcohols by methanesulfonate ion with inversion of configuration is described. This work provides an alternative to the Mitsunobu reaction that avoids the use of azodicarboxylates and generation of hydrazine and phosphine oxide byproducts. This transformation is shown to be compatible with a range of functionality. A cyclopropenone scavenge
表征谱图
-
氢谱1HNMR
-
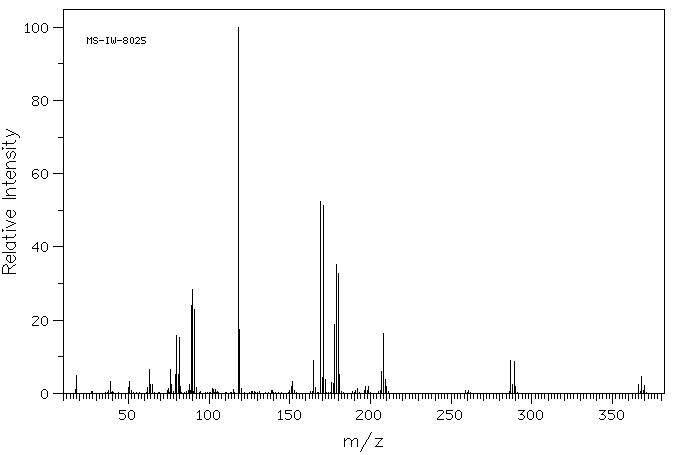
质谱MS
-
碳谱13CNMR
-
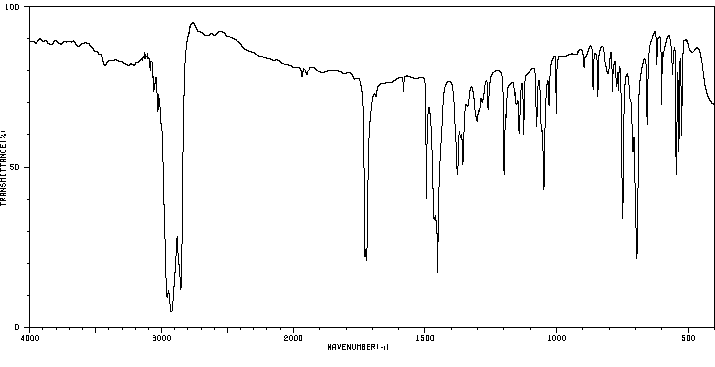
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2Z)-1,3-二苯基-2-丙烯-1-酮,2-丙烯-1-酮,1,3-二苯基-,(2Z)-
龙血素D
龙血素A
龙血素 B
黄色当归醇F
黄色当归醇B
黄腐醇; 黄腐酚
黄腐醇 D; 黄腐酚 D
黄腐酚B
黄腐酚
黄腐酚
黄卡瓦胡椒素 C
高紫柳查尔酮
阿普非农
阿司巴汀
阿伏苯宗
金鸡菊查耳酮
邻肉桂酰苯甲酸
达泊西汀杂质25
豆蔻明
补骨脂色烯查耳酮
补骨脂查耳酮
补骨脂呋喃查耳酮
补骨脂乙素
蜡菊亭; 4,2',4'-三羟基-6'-甲氧基查耳酮
苯酚,4-[3-(2-羟基苯基)-1-苯基丙基]-2-(3-苯基丙基)-
苯磺酰胺,N-[4-[3-(3-羟基苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,N-[3-[3-(4-羟基-3-甲氧苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,4-甲氧基-N,N-二甲基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯化,4,5-二甲氧基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯,4-甲氧基-3-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯甲醇,4-甲氧基-a-[2-(4-甲氧苯基)乙烯基]-
苯甲酸-[4-(3-氧代-3-苯基-丙烯基)-苯胺]
苯甲酸,3-[3-(4-溴苯基)-1-羰基-2-丙烯基]-4-羟基-
苯甲酰(2-羟基苯酰)甲烷
苯甲腈,4-(1-羟基-3-羰基-3-苯基丙基)-
苯基[2-(1-萘基)乙烯基]甲酮
苯基-(三苯基-丙-2-炔基)-醚
苯基-(2-苯基-2,3-二氢-苯并噻唑-2-基)-甲酮
苯亚甲基苯乙酮
苯乙酰腈,a-(1-氨基-2-苯基亚乙基)-
苯丙酸,a-苯甲酰-b-羰基-,苯基(苯基亚甲基)酰肼
苯,1-(2,2-二甲基-3-苯基丙基)-2-甲基-
苏木查耳酮
苄桂哌酯
苄基(4-氯-2-(3-氧代-1,3-二苯基丙基)苯基)氨基甲酸酯
芦荟提取物
腈苯唑
胀果甘草宁C
聚磷酸根皮酚