1,5-二甲基萘 | 571-61-9
中文名称
1,5-二甲基萘
中文别名
1,5-二甲基萘
英文名称
1,5-dimethylnaphthalene
英文别名
1,5-Dimethyl-naphthalin;Dimethylnaphthalin
CAS
571-61-9
化学式
C12H12
mdl
MFCD00004038
分子量
156.227
InChiKey
SDDBCEWUYXVGCQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:265.0 °C
-
熔点:82.0 °C
-
溶解度:1.75e-05 M
-
蒸汽压力:0.01 mmHg
-
亨利常数:3.50e-04 atm-m3/mole
-
保留指数:1398.4;1398;1440;1440;1448;1421;1420;1420.7;1430;245
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2902909090
-
储存条件:贮存: 将密器密封后,储存在密封的主容器中,并放置于阴凉、干燥处。
SDS
Section I.Chemical Product and Company Identification
Chemical Name 1,5-Dimethylnaphthalene
Portland OR
Synonym Not available.
Chemical Formula C10H6(CH3)2
CAS Number 571-61-9
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
1,5-Dimethylnaphthalene 571-61-9 Min. 99.0(GC) Not available. Not available.
Section III. Hazards Identification
Acute Health Effects Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening,
or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when
handling this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
Inhalation If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the
toxic material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Auto-Ignition Not available.
Flammability May be combustible at high temperature.
Flammable Limits
Flash Points Not available.
Not available.
Combustion Products These products are toxic carbon oxides (CO, CO 2).
Fire Hazards
Not available.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
and Instructions LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
1,5-Dimethylnaphthalene
Section VI. Accidental Release Measures
Spill Cleanup Irritating material.
Instructions Use a shovel to put the material into a convenient waste disposal container. Finish cleaning the spill by rinsing any
contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
Handling and Storage IRRITANT. Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the container and store in
a dry, cool place. Avoid excessive heat and light. Do not breathe dust.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection Splash goggles. Lab coat. Dust respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult
a specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solubility
Physical state @ 20°C Solid. (White crystalline powder.) Not available.
Not available.
Specific Gravity
Molecular Weight 156.23 Partition Coefficient Not available.
Boiling Point 265 to 266°C (509 to 510.8°F) Vapor Pressure Not applicable.
Melting Point 80 to 82°C (176 to 179.6°F) Vapor Density Not available.
Not available. Volatility Not available.
Refractive Index
Critical Temperature Odor Not available.
Not available.
Not available. Not available.
Viscosity Taste
Section X. Stability and Reactivity Data
This material is stable if stored under proper conditions. (See Section VII for instructions)
Stability
Conditions of Instability
Avoid excessive heat and light.
Incompatibilities
Reactive with strong oxidizing agents.
Section XI. Toxicological Information
RTECS Number Not available.
Routes of Exposure Eye Contact. Ingestion. Inhalation.
Not available.
Toxicity Data
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
Acute Toxic Effects
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening,
or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when
handling this compound.
Continued on Next Page
1,5-Dimethylnaphthalene
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
Waste Disposal
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
PIN Number Not applicable.
Proper Shipping Name
Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not available.
(Canada)
EINECS Number (EEC) 209-338-5
EEC Risk Statements R36/37/38- Irritating to eyes, respiratory system and skin.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,4-二甲基萘 1,4-dimethylnaphthalene 571-58-4 C12H12 156.227 1,8-二甲基萘 1,8-dimethylnaphthalene 569-41-5 C12H12 156.227 二甲基萘 dimethyl-1,2 naphtalene 573-98-8 C12H12 156.227 1,5-双(氯甲基)萘 1,5-bis(chloromethyl)naphthalene 1733-76-2 C12H10Cl2 225.117 2,6-二甲基萘 2.6-dimethylnaphthalene 581-42-0 C12H12 156.227 2-甲基萘 2-Methylnaphthalene 91-57-6 C11H10 142.2 (氯甲基)萘 1-Chloromethylnaphthalene 86-52-2 C11H9Cl 176.645 2,3-二甲基萘 2,3-dimethylnaphthalene 581-40-8 C12H12 156.227 —— 1,5-dimethyl-3,7-di-tert-butylnaphthalene 79750-37-1 C20H28 268.442 1-溴-5-甲基萘 1-bromo-5-methylnaphthalene 20366-59-0 C11H9Br 221.096 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,7-二甲基萘 1,7-dimethylnaphthalene 575-37-1 C12H12 156.227 三甲基萘 1,4,5-trimethylnaphthalene 2131-41-1 C13H14 170.254 甲基萘 1-Methylnaphthalene 90-12-0 C11H10 142.2 1,2,5-三甲基萘 1,2,5-trimethylnaphthalene 641-91-8 C13H14 170.254 1,6-二甲基萘 1,6-dimethylnaphthalene 575-43-9 C12H12 156.227 1,3-二甲基萘 1,3-dimethylnaphthalene 575-41-7 C12H12 156.227 —— (5-methylnaphth-1-yl)methanol 65755-14-8 C12H12O 172.227 —— 5-methyl-1-naphthaldehyde 104306-72-1 C12H10O 170.211 1,5-双(羟甲基)萘 1,5-bis(hydroxymethyl)naphthalene 54835-54-0 C12H12O2 188.226 —— 1,5-Divinylnaphthalene 46263-17-6 C14H12 180.249 1,5-双(氯甲基)萘 1,5-bis(chloromethyl)naphthalene 1733-76-2 C12H10Cl2 225.117 —— 1-Brommethyl-5-methyl-naphthalin 21674-39-5 C12H11Br 235.123 1,5-双(溴甲基)萘 1,5-di(bromomethyl)naphthalene 21646-18-4 C12H10Br2 314.019 2,6-二甲基萘 2.6-dimethylnaphthalene 581-42-0 C12H12 156.227 2-甲基萘 2-Methylnaphthalene 91-57-6 C11H10 142.2 2,7-二甲基萘 2,7-dimethylnaphthalene 582-16-1 C12H12 156.227 —— 2-Chlor-1,5-dimethylnaphthalin 62956-42-7 C12H11Cl 190.672 3,7-二溴-1,5-二甲基萘 3,7-dibromo-1,5-dimethylnaphthalene 111650-47-6 C12H10Br2 314.019 —— 1,5-Distyrylnaphthalene 80236-27-7 C26H20 332.445 —— 2,6-dibromo-1,5-dimethylnaphthalene 111650-45-4 C12H10Br2 314.019 —— 4,8-Dimethylnaphthalen-1-ol 142209-79-8 C12H12O 172.227 —— naphthalene-1,5-diacetic acid 25178-67-0 C14H12O4 244.247 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:Mechanisms of photochemical reactions in solution. LXXXI. Photocyclization of 1,8-divinylnaphthalene. New method for determining the multiplicity of excited state intermediates摘要:DOI:10.1021/ja00832a020
-
作为产物:参考文献:名称:Pozdnyakovich, Yu. V., Journal of Organic Chemistry USSR (English Translation), 1981, p. 1386 - 1387摘要:DOI:
-
作为试剂:参考文献:名称:手性6-苯基-2,3-双亚甲基甲氧基羰基-[1,4]-二恶烷作为设计合成子,可高效且短时间合成光学纯的2,6-二恶双环[3.3.0]辛烷-3,7-二酮摘要:通过8的PET环化反应合成的手性6-苯基-2,3-双亚甲基甲氧基羰基-[1,4]-二恶烷已被用作设计合成子,可以高效,短时合成光学纯的2,6-二恶双环[3.3]。 .0]辛烷-3,7-二酮。DOI:10.1016/j.tetlet.2005.11.116
文献信息
-
NOVEL NAPHTHYLENYL COMPOUNDS FOR LONG-ACTING INJECTABLE COMPOSITIONS AND RELATED METHODS申请人:Alkermes Pharma Ireland Limited公开号:US20200016151A1公开(公告)日:2020-01-16The present invention provides compounds useful for the treatment of opioid dependence, alcohol dependence, alcohol use disorder, or the prevention of relapse to opioid dependence in a subject in need thereof. Related pharmaceutical compositions and methods are also provided herein.
-
Palladium-Catalysed Direct Cross-Coupling of Organolithium Reagents with Aryl and Vinyl Triflates作者:Carlos Vila、Valentín Hornillos、Massimo Giannerini、Martín Fañanás-Mastral、Ben L. FeringaDOI:10.1002/chem.201404398日期:2014.10.6A palladium‐catalysed cross‐coupling of organolithium reagents with aryl and vinyl triflates is presented. The reaction proceeds at 50 or 70 °C with short reaction times, and the corresponding products are obtained with moderate to high yields, with a variety of alkyl and (hetero)aryl lithium reagents.
-
Structures and photoactivation of the charge-transfer complexes of bis(arene)iron(II) dications with ferrocene and arene donors作者:R. E. Lehmann、Jay K. KochiDOI:10.1021/ja00002a018日期:1991.1crystals with the isoelectronic bis(arene)iron(II) dication, in which X-ray crystallography establishes the alternate (heterosoric) stacking of sandwich structures comprising the donor-acceptor pairs. In acetonitrile, the 1:1 complex (Cpsub 2}Fe, Arsub 2}Fesup 2+}) shows a broad absorption band centered at lambda}sub max} approximately} 630 nm. Bis(arene)iron(II) acceptors also form highly colored
-
Metal-free oxidation of aromatic carbon–hydrogen bonds through a reverse-rebound mechanism作者:Changxia Yuan、Yong Liang、Taylor Hernandez、Adrian Berriochoa、Kendall N. Houk、Dionicio SiegelDOI:10.1038/nature12284日期:2013.7.11functions as a selective oxidant for the transformation of arenes to phenols under mild conditions. Although the reaction proceeds through a radical mechanism, aromatic C–H bonds are selectively oxidized in preference to activated –H bonds. Notably, a wide array of functional groups are compatible with this reaction, and this method is therefore well suited for late-stage transformations of advanced碳氢 (C-H) 键氧化方法在合成有机化学中具有重要作用,可提供最终目标分子所需的功能或促进后续化学转化。已经描述了几种氧化脂肪族 C-H 键的方法,大大简化了复杂分子的合成。然而,在温和条件下选择性氧化芳族 C-H 键,尤其是在具有不同官能团的取代芳烃的情况下,仍然是一个挑战。芳烃的直接羟基化最初是通过使用强布朗斯台德或路易斯酸与超化学计量当量的氧化剂介导亲电芳香取代反应来实现的,这显着限制了反应范围。由于这些反应的产物比起始材料更具反应性,因此过度氧化通常是一个竞争过程。已经开发了过渡金属催化芳烃的 C-H 氧化,有或没有导向基团,改进了酸介导的过程;但是,需要贵金属。在这里,我们证明过氧化邻苯二甲酰作为选择性氧化剂在温和条件下将芳烃转化为酚类。尽管反应通过自由基机制进行,但芳香族 C-H 键优先于活化的 -H 键被选择性氧化。值得注意的是,大量的官能团与该反应相容,因此该方法非常适合
-
The Oxidation of Hydrophobic Aromatic Substrates by Using a Variant of the P450 Monooxygenase CYP101B1作者:Md. Raihan Sarkar、Joel H. Z. Lee、Stephen G. BellDOI:10.1002/cbic.201700316日期:2017.11.2What a bind! The CYP101B1 enzyme from the bacteria N. aromaticivorans has been engineered to better bind and oxidise hydrophobic aromatic substrates. His85 was identified by sequence alignment as a potential active-site residue. The H85F variant improved activity and offered unusual selectivity options.
表征谱图
-
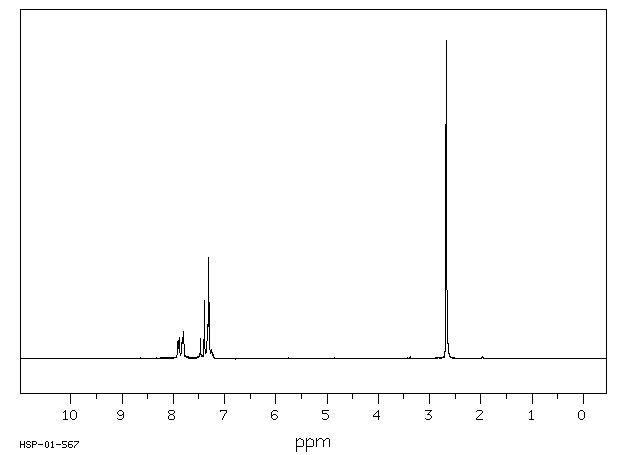
氢谱1HNMR
-
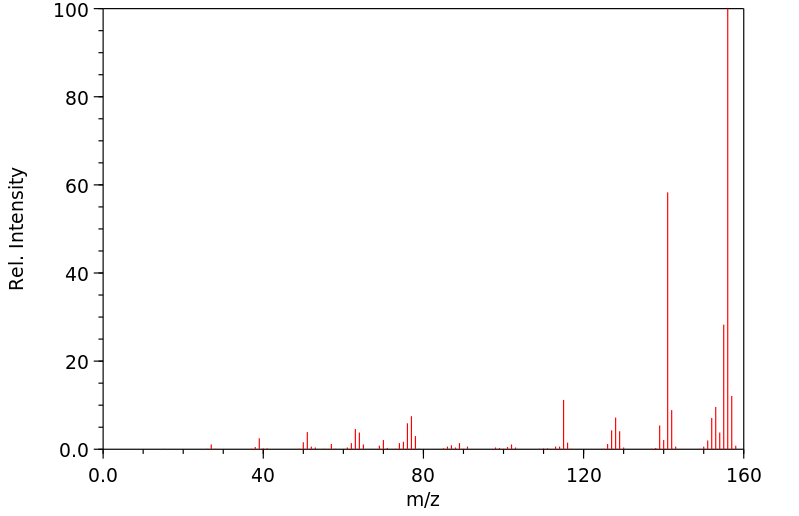
质谱MS
-
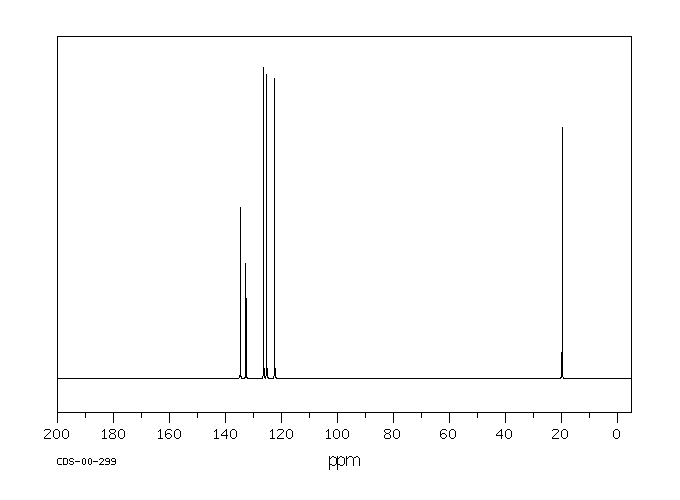
碳谱13CNMR
-
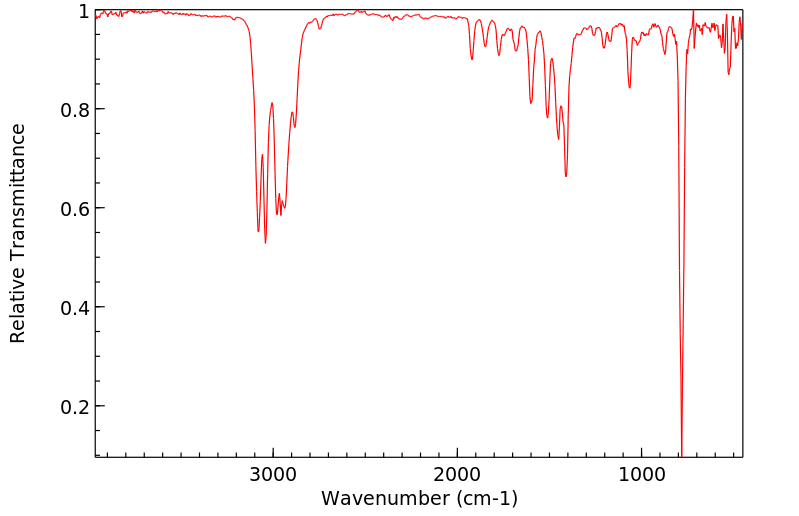
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮