1-(2-氯苯基)-3-苯基-2-硫脲 | 1932-36-1
中文名称
1-(2-氯苯基)-3-苯基-2-硫脲
中文别名
——
英文名称
1-(2-chloro-phenyl)-3-phenyl-thiourea
英文别名
1-(2-Chlorophenyl)-3-phenylthiourea
CAS
1932-36-1
化学式
C13H11ClN2S
mdl
MFCD00022109
分子量
262.763
InChiKey
GJXBIVMCTDXUKR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:156°C
-
沸点:371.4±44.0 °C(Predicted)
-
密度:1.385±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:56.2
-
氢给体数:2
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
海关编码:2930909090
-
危险类别:IRRITANT
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(2-氯苯基)-3-苯基脲 1-(2-chlorophenyl)-3-phenylurea 2989-99-3 C13H11ClN2O 246.696 —— o-chlorophenylthiocarbamic acid O-methyl ester 20399-40-0 C8H8ClNOS 201.677
反应信息
-
作为反应物:描述:1-(2-氯苯基)-3-苯基-2-硫脲 在 盐酸 、 sodium ethanolate 、 sodium acetate 、 sodium nitrite 作用下, 以 乙醇 为溶剂, 反应 10.0h, 生成 (5E)-1-(2-chlorophenyl)-3-phenyl-5-(phenylhydrazinylidene)-2-sulfanylidene-1,3-diazinane-4,6-dione参考文献:名称:Singh, S.; Behl, C. K., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1980, vol. 19, # 7, p. 625 - 626摘要:DOI:
-
作为产物:参考文献:名称:硫脲衍生物,结构简单但高效的酶抑制剂和汞传感器摘要:在这项研究中,六种不对称硫脲衍生物,1-异丁基-3-环己基硫脲 ( 1 )、1-叔丁基-3-环己基硫脲( 2 )、1-(3-氯苯基)-3-环己基硫脲( 3 )、1-( 1,1-二丁基)-3-苯基硫脲(4)、1-(2-氯苯基)-3-苯基硫脲(5)和1-(4-氯苯基)-3-苯基硫脲(6)在实验室有氧条件下得到状况。化合物3和4是结晶的,它们的结构由它们的单晶确定。化合物3为单斜晶系,空间群为 P2 1 /n 而化合物4是三角的,空间群R 3 :H。化合物(1 - 6)它们对乙酰胆碱酯酶和丁酰胆碱酯酶抗胆碱酯酶活性(分别为以下简称为,乙酰胆碱酯酶和BChE的)进行了测试。还研究了使用荧光光谱技术测定致命有毒金属(汞)作为传感探针的潜力(所有化合物)。化合物3对AChE和BChE表现出更好的酶抑制IC 50值为50和60 μg/mL,对接评分分别为-10.01和-8.04 kJ/mol。该化合物在荧光研究期间也表现出中等灵敏度。DOI:10.3390/molecules26154506
文献信息
-
Inhibition of Adipogenesis by Thiourea Derivatives作者:Hina Siddiqui、Sarah Shafi、Farah Mukhtar、Asma Ejaz、Atta ur-Rahman、M. Iqbal ChoudharyDOI:10.2174/1573406413666171120161208日期:2018.7.6adipogenesis as the treatment for obesity. METHOD We describe here, the synthesis, and anti-adipogenic activity of thiourea derivatives 1-14. These derivatives were synthesized by the reactions of phenyl and pentafluorophenyl isothiocyanate with different aromatic amines. Pure compounds 1-14 were evaluated for their in vitro antiadipogenesis activity employing 3T3-L1 cells lines. RESULTS Compounds 1-3, 5-9, and背景技术肥胖是具有2型糖尿病,高血压,CVD等固有风险的主要健康问题之一。脂肪形成是肥胖过程中的主要贡献者。抑制脂肪细胞分化是治疗肥胖症的关键方法之一。目的发现新的脂肪形成抑制剂作为肥胖症的治疗方法。方法我们在这里描述了硫脲衍生物1-14的合成和抗脂肪形成活性。这些衍生物是通过苯基和五氟苯基异硫氰酸酯与不同的芳族胺反应合成的。使用3T3-L1细胞系评估纯化合物1-14的体外抗脂肪形成活性。结果化合物1-3、5-9和11-14可以显着抑制前脂肪细胞向脂肪细胞的分化,这是通过对细胞染色来测定的,并通过形态学检查。化合物10(1-(4“-氯苯基)-3-(五氟苯基)-硫脲)表现出对脂肪细胞分化的有效抑制作用,IC50 = 740.00±2.36 nM,比标准表没食子儿茶素没食子酸酯(IC50 = 16.73± 1.34μM)和姜黄素(IC50 = 18.62±0.74μM)。所有其他化合物均显示出
-
Synthesis of 1,2,4-oxadiazolidines <i>via</i> [3+2] cycloaddition of nitrones with carbodiimides作者:Yuan Chen、Liuting Fuyue、Gangqiang Wang、Hang Wang、Chun Lu、Haibing Guo、Chiara St. Amant、Shaofa Sun、Yalan XingDOI:10.1039/c9nj00030e日期:——An efficient [3+2] cycloaddition of nitrones and carbodiimides has been developed. This 1,3-dipolar cycloaddition features high regioselectivity, mild and metal-free conditions, excellent functional group compatibility, and a broad substrate scope. The X-ray structures of two regio-isomers confirmed the regioselectivity of this transformation.
-
o-Iodoxybenzoic Acid Mediated Oxidative Desulfurization of 1,3-Disubstituted Thioureas to Carbodiimides作者:Krishnacharya Akamanchi、Pramod Chaudhari、Prasad DangateDOI:10.1055/s-0030-1259072日期:2010.12An efficient and mild oxidative desulfurization procedure using o-iodoxybenzoic acid has been developed for the synthesis of carbodiimides starting from easily synthesizable 1,3-disubstituted thioureas.
-
Preparation of 2-Azido-1-Substituted-1 <i>H</i>-Benzo[<i>d</i>]imidazoles Using a Copper-Promoted Three-Component Reaction and Their Further Conversion into 2-Amino and 2-Triazolyl Derivatives作者:Tamminana Ramana、Tharmalingam PunniyamurthyDOI:10.1002/chem.201202215日期:2012.10.15Multicomponent reaction: 2‐Azido‐1‐substituted‐1H‐benzo[d]imidazoles were prepared using a copper‐catalyzed three‐component reaction involving 2‐bromoaniline derivatives, isothiocyanates, and sodium azide. The reaction conditions were mild and the scope was broad. The azido compounds were transformed into their 2‐amino and 2‐triazolyl derivatives using copper‐mediated reduction and cycloaddition, respectively
-
Tandem regioselective synthesis of tetrazoles and related heterocycles using iodine作者:Ramesh Yella、Nilufa Khatun、Saroj Kumar Rout、Bhisma K. PatelDOI:10.1039/c0ob01007c日期:——carbodiimides). The in situ generated heterocumulene on subsequent treatment with sodium azide at room temperature gave corresponding tetrazoles. The product regioselectivity for unsymmetrical 1,3-disubstituted thioureas was found to be correlated with the basicities (pKa's) of the parent amines attached to the thiourea. Aryl-sec-alkyl unsymmetrical thioureas gave thioamido guanidino products rather than the 5-aminotetrazoles
表征谱图
-
氢谱1HNMR
-
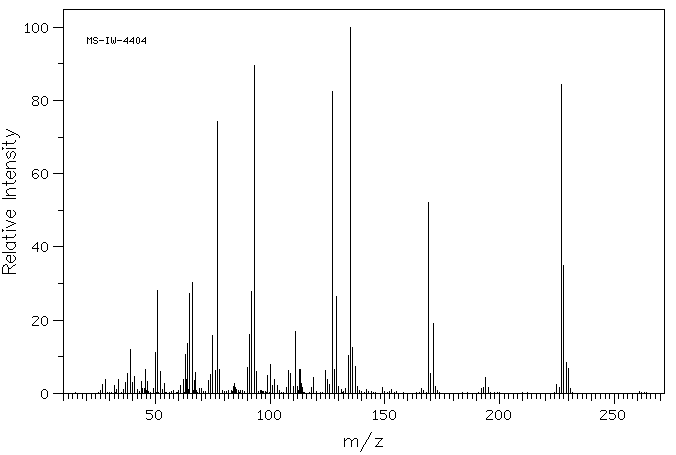
质谱MS
-
碳谱13CNMR
-
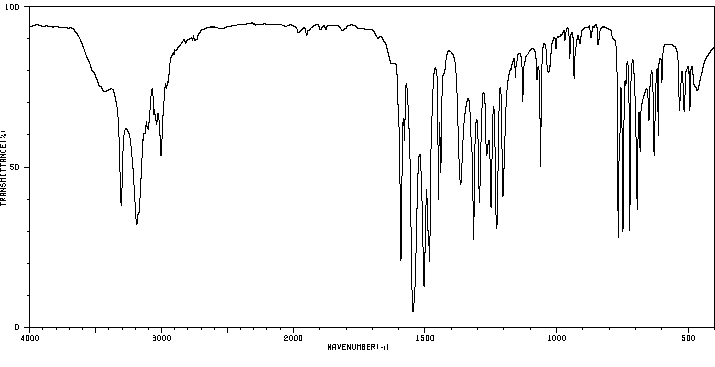
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫