月桂酸丙酯 | 3681-78-5
中文名称
月桂酸丙酯
中文别名
——
英文名称
n-propyl laurate
英文别名
propyl laurate;propyl dodecanoate
CAS
3681-78-5
化学式
C15H30O2
mdl
——
分子量
242.402
InChiKey
FTBUKOLPOATXGV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:4.2°C (estimate)
-
沸点:285.23°C (estimate)
-
密度:0.86 g/mL at 25 °C(lit.)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
LogP:6.110
-
保留指数:1676;1669
计算性质
-
辛醇/水分配系数(LogP):6.2
-
重原子数:17
-
可旋转键数:13
-
环数:0.0
-
sp3杂化的碳原子比例:0.93
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915900090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:2-8°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Propyl laurate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Propyl laurate
CAS number: 3681-78-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C15H30O2
Molecular weight: 242.4
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Propyl laurate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Propyl laurate
CAS number: 3681-78-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C15H30O2
Molecular weight: 242.4
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:N,N-二取代酰胺和异丙基酯直接转化为腈摘要:通过用氢化二异丁基铝处理,然后用氨水中的分子碘处理,可以将各种N,N-二甲基酰胺,N-甲氧基-N-甲基酰胺和异丙基酯以良好至中等的收率平稳地转化为相应的腈。本反应是新颖的一锅法和实用方法,其通过分别形成半胱氨酸O -AlBu 2和半缩醛O -AlBu 2来将N,N-二取代酰胺和异丙酯转化为腈。DOI:10.1016/j.tet.2011.04.008
-
作为产物:描述:butyryl dodecanoyl peroxide 以1%的产率得到参考文献:名称:FELDHUES, M.;SCHAEFER, H. J., TETRAHEDRON, 1985, 41, N 19, 4195-4212摘要:DOI:
文献信息
-
METHOD FOR CONTINUOUSLY PREPARING CARBOXYLIC ACID ESTER申请人:CHINA PETROCHEMICAL DEVELOPMENT CORPORATION公开号:US20130303796A1公开(公告)日:2013-11-14A method for continuously preparing a carboxylic acid ester is disclosed. In the method of the present invention, a vertical reactor is filled with a solid catalyst, a carboxylic acid and an alcohol are introduced into a lower part of the vertical reactor, esterification is performed to form an esterized mixture, the esterized mixture is output from an upper part of the vertical reactor, and distillation is performed to isolate the carboxylic acid ester. The method of the present invention is simple, easily controlled and environmental friendly, and has significantly high conversion rate and selectivity.
-
Process for Production of Alkyl Tin Alkoxide Compound, and Process for Production of Carbonic Acid Ester Using the Compound申请人:Shinohata Masaaki公开号:US20100292496A1公开(公告)日:2010-11-18The present invention provides a process for producing: a compound represented by XOR 2 ; a dialkyl tin dialkoxide compound having one tin atom, two Sn—R 1 bonds and two Sn—OR 2 bonds; and/or a tetraalkyl dialkoxy distannoxane compound having one Sn—O—Sn bond, in which each tin atom of the tetraalkyl dialkoxy distannoxane compound has two Sn—R 1 bonds and one Sn—OR 2 bond, the process comprising reacting in the absence of a catalyst at least one alkyl tin compound selected from the group consisting of i) and ii) below: i) a dialkyl tin compound having one tin atom, two Sn—R 1 (wherein R 1 represents an alkyl group) bonds, and two Sn—OX bonds (wherein OX is a group in which HOX that is a conjugate acid of OX is a Bronsted acid having a pKa of from 0 to 6.8); and ii) a tetraalkyl distannoxane compound having one Sn—O—Sn bond, in which each tin atom of the tetraalkyl distannoxane compound has two Sn—R 1 bonds and one Sn—OX bond (wherein OX is a group in which HOX that is a conjugate acid of OX is a Bronsted acid having a pKa of from 0 to 6.8); and a carbonic acid ester represented by R 2 OCOOR 2 (wherein R 2 represents a linear or branched, saturated or unsaturated hydrocarbon group, a hydrocarbon group having a saturated or unsaturated cyclic hydrocarbon substituent, or a Y—CH 2 — group (wherein Y represents an alkyl polyalkylene group, an aromatic group or a cyclic saturated or unsaturated alkylene ether group)), and/or an alcohol represented by R 2 OH (wherein R 2 is the same as defined above).本发明提供了一种生产过程:产生一个由XOR表示的化合物;具有一个锡原子、两个Sn—R1键和两个Sn—OR2键的二烷基锡二烷氧化合物;和/或具有一个Sn—O—Sn键的四烷基二烷氧基二锡烷氧化合物,其中四烷基二烷氧基二锡烷氧化合物的每个锡原子具有两个Sn—R1键和一个Sn—OR2键,所述过程包括在缺乏催化剂的情况下反应以下所述组中选择的至少一种烷基锡化合物: i) 具有一个锡原子、两个Sn—R1(其中R1代表烷基基团)键和两个Sn—OX键(其中OX是HOX的共轭酸,HOX是具有从0到6.8的pKa的Bronsted酸的群)的二烷基锡化合物;和 ii) 具有一个Sn—O—Sn键的四烷基二锡烷氧化合物,其中四烷基二锡烷氧化合物的每个锡原子具有两个Sn—R1键和一个Sn—OX键(其中OX是HOX的共轭酸,HOX是具有从0到6.8的pKa的Bronsted酸的群);和 由R2OCOOR2(其中R2代表线性或支链、饱和或不饱和碳氢基团、具有饱和或不饱和环烃取代基的碳氢基团,或Y—CH2—基团(其中Y代表烷基多聚烯基基团、芳香基团或环状饱和或不饱和烷基醚基团))表示的碳酸酯;和/或 由R2OH(其中R2与上述定义相同)表示的醇。
-
Tunable aryl imidazolium ionic liquids (TAIILs) as environmentally benign catalysts for the esterification of fatty acids to biodiesel fuel作者:Mayur Thul、Amit Pantawane、Wesley Lin、Yi-Jyun Lin、Po-Fang Su、Shao-An Tseng、Hsin-Ru Wu、Wen-Yueh Ho、Shun-Yuan LuoDOI:10.1016/j.catcom.2020.106243日期:2021.1Herein, we describe the synthesis of tunable aryl imidazolium ionic liquid catalysts and tested for esterification of fatty acids to biodiesel. In this work, six tunable aryl imidazolium ionic liquids (TAIILs) 1a-1f were prepared. These ionic liquids were used as the economical and reusable catalysts for the synthesis of biodiesel fuels. The reaction has been preceded in a monophase at 80 °C for 4 h
-
Mild Esterification and Transesterification of Carboxylic Acids Catalyzed by Tetracyanoethylene and Dicyanoketene Dimethyl Acetal作者:Yukio Masaki、Nobuyuki Tanaka、Tsuyoshi MiuraDOI:10.1246/cl.1997.55日期:1997.1A π-acid tetracyanoethylene (TCNE) and its derivative dicyanoketene dimethyl acetal (DCKDMA) were found to catalyze esterification of lauric acid with various types of alcohols. This mathod was successfully applied to methyl esterification of a variety of carboxylic acids including aromatic, α,β-unsaturated, α-hydroxy, and N-Cbz and N-Boc-protected α-amino acids without racemization at the range from
-
Electrophysiological Responses of Bactrocera kraussi (Hardy) (Tephritidae) to Rectal Gland Secretions and Headspace Volatiles Emitted by Conspecific Males and Females作者:Sally Noushini、Soo Jean Park、Jeanneth Perez、Danielle Holgate、Vivian Mendez、Ian M. Jamie、Joanne F. Jamie、Phillip W. TaylorDOI:10.3390/molecules26165024日期:——Pheromones are biologically important in fruit fly mating systems, and also have potential applications as attractants or mating disrupters for pest management. Bactrocera kraussi (Hardy) (Diptera: Tephritidae) is a polyphagous pest fruit fly for which the chemical profile of rectal glands is available for males but not for females. There have been no studies of the volatile emissions of either sex信息素在果蝇交配系统中具有重要的生物学意义,并且还具有作为害虫管理的引诱剂或交配干扰剂的潜在应用。 Bactrocera kraussi (Hardy)(双翅目:实蝇科)是一种杂食性害虫果蝇,其直肠腺的化学特征仅适用于雄性,但不适用于雌性。目前还没有关于性别挥发性排放或对这些化合物的电生理反应的研究。本研究(i)通过气相色谱-质谱(GC-MS)建立了两性B. kraussi直肠腺内容物和挥发物的化学特征,(ii)评估了气相色谱对已鉴定化合物的检测 -触角电图检测(GC-EAD)和触角电图检测(GC-EPD)。在雄性B. kraussi 的直肠腺中鉴定出 16 种化合物,在雌性 B. kraussi的直肠腺中鉴定出 29 种化合物。在这些化合物中,有 5 种在雄性的顶部空间中检测到,13 种在雌性的顶部空间中检测到。 GC-EPD 测定记录了两性针对 ( E , E )-2,8-二甲基-1,7-二氧螺[5
表征谱图
-
氢谱1HNMR
-
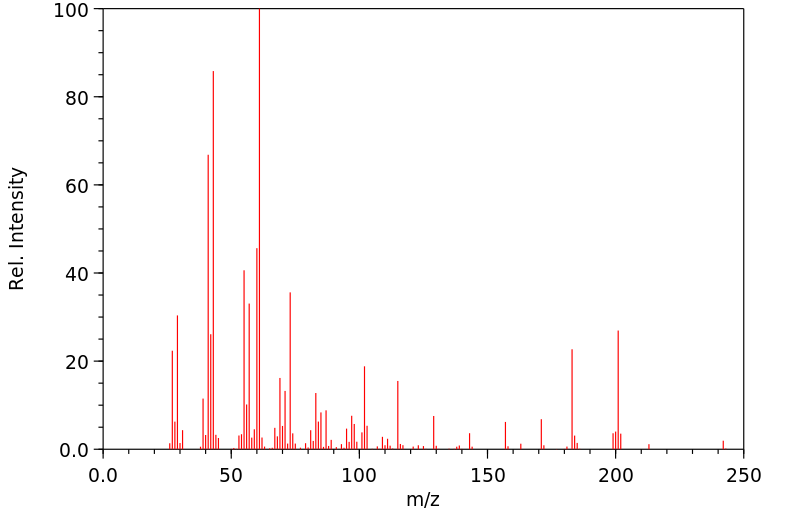
质谱MS
-
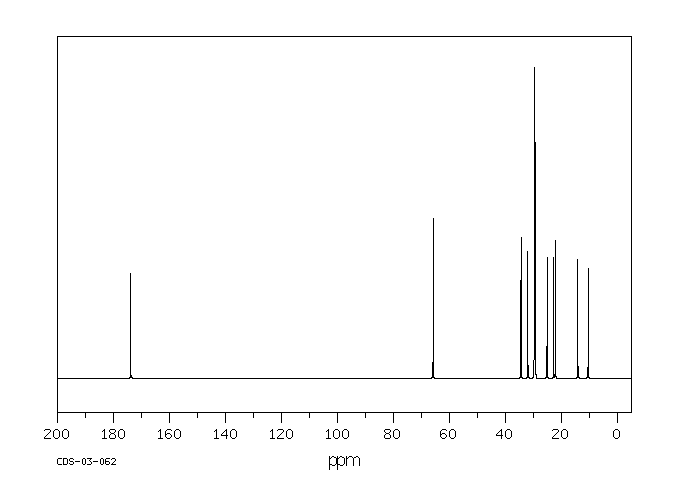
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯