1-(4-溴苯基)-3-(4-氯苯基)丙-2-烯-1-酮 | 126443-16-1
分子结构分类
中文名称
1-(4-溴苯基)-3-(4-氯苯基)丙-2-烯-1-酮
中文别名
——
英文名称
(E)-1-(4-bromophenyl)-3-(4-chlorophenyl)prop-2-en-1-one
英文别名
ACETOPHENONE, 4'-BROMO-2-(p-CHLOROBENZYLIDENE)-
CAS
126443-16-1
化学式
C15H10BrClO
mdl
——
分子量
321.601
InChiKey
HYCQSDMDGCPEPI-XCVCLJGOSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-溴苯乙酮 para-bromoacetophenone 99-90-1 C8H7BrO 199.047
反应信息
-
作为反应物:描述:1-(4-溴苯基)-3-(4-氯苯基)丙-2-烯-1-酮 在 sodium hydride 、 sodium hydroxide 作用下, 以 甲醇 、 乙醇 、 水 为溶剂, 反应 6.0h, 生成 2-(3-(4-bromophenyl)-1-(4-chlorophenyl)-3-oxopropyl)malonic acid参考文献:名称:2-(3-Oxo-1,3-diphenylpropyl)malonic Acids as Potent Allosteric Ligands of the PIF Pocket of Phosphoinositide-Dependent Kinase-1: Development and Prodrug Concept摘要:The protein kinase C-related kinase 2 (PRK2)interacting fragment (PIF) pocket of phosphoinositide-dependent kinase-1 (PDK1) was proposed as a novel target site for allosteric modulators. In the present work, We describe the design, synthesis, and structure-activity relationship of a series of 2-(3-oxo-1,3- diphenylprapyl)malonic acids as potent allosteric activators binding, to the PIF pocket: Some congeners displayed AC(50) values for PDK1 activation in the submicromolar range. The potency of the best compounds to stabilize PDK1 in a thermal stability shift assay was in the same order Of magnitude as that of the PIF pocket binding peptide PIFtide, suggesting comparable binding affinities to the pm pocket, The crystal structure of PDK1 in complex with compound 4h revealed that additional ionic interactions are mainly responsible for the increased potency compared to the monocarboxylate analogues. Notably, several compounds displayed high selectivity for PDK1 Employing a prodrug strategy, we were able to corrroborate the novel mechanism of action in cells.DOI:10.1021/jm3010477
-
作为产物:参考文献:名称:四正丁基溴化铵:温和条件下酒精氧化的一种简单而有效的有机催化剂摘要:首次鉴定出一种简单而有效的有机催化体系,该体系以5 mol%的四正丁基溴化铵(TBAB)为催化剂。该有机催化体系可与多种具有各种催化反应性基团的苄基/烯丙基醇相容。此外,它对仲苄醇的选择性优于脂族醇,对4-(1-羟乙基)苄醇的伯苄醇位具有良好的选择性。因此,与该TBAB有机催化体系相关的反应条件的简单,高效,选择性和温和的特征表明其在合成化学中的广泛应用的潜力。DOI:10.1002/adsc.201400100
文献信息
-
[EN] ALLOSTERIC PROTEIN KINASE MODULATORS<br/>[FR] MODULATEURS DE PROTÉINE KINASE ALLOSTÉRIQUE申请人:UNIV SAARLAND公开号:WO2010043711A1公开(公告)日:2010-04-22The invention provides specific small molecule compounds that allosterically regulate the activity or modulate protein-protein interactions of AGC protein kinases and the Aurora family of protein kinases, methods for their production, pharmaceutical compositions comprising same, and their use for preparing medicaments for the treatment and prevention of diseases related to abnormal activities of AGC protein kinases or of protein kinases of the Aurora family.该发明提供特定的小分子化合物,这些化合物能够变构调节AGC蛋白激酶的活性或调节Aurora家族蛋白激酶的蛋白-蛋白相互作用,提供这些化合物的制备方法,包含这些化合物的药物组合物,以及它们用于制备治疗和预防与AGC蛋白激酶或Aurora家族蛋白激酶异常活动相关疾病的药物的应用。
-
ALLOSTERIC PROTEIN KINASE MODULATORS申请人:Engel Matthias公开号:US20120046307A1公开(公告)日:2012-02-23The invention provides specific small molecule compounds that allosterically regulate the activity or modulate protein-protein interactions of AGC protein kinases and the Aurora family of protein kinases, methods for their production, pharmaceutical compositions comprising same, and their use for preparing medicaments for the treatment and prevention of diseases related to abnormal activities of AGC protein kinases or of protein kinases of the Aurora family.本发明提供了特定的小分子化合物,它们通过变构调节AGC蛋白激酶的活性或调节Aurora家族蛋白激酶的蛋白质-蛋白质相互作用,其生产方法,包含该化合物的药物组合物,以及它们用于制备治疗和预防与AGC蛋白激酶或Aurora家族蛋白激酶异常活动相关疾病的药物的应用。
-
Regioselective Synthesis of 1,3,5-Trisubstituted Pyrazoles作者:Vidya G. Desai、Pooja C. Satardekar、Sampada Polo、Kashinath DhumaskarDOI:10.1080/00397911.2010.531492日期:2012.3.15Abstract A new and a simple approach toward synthesis of 1,3,5-trisubstituted pyrazoles from chalcone arylhydrazones via oxidative cyclization has been achieved. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone was successfully used as an oxidizing agent to give excellent yields of pyrazoles. GRAPHICAL ABSTRACT
-
Michael-Michael Ring Closure Reaction of Benzyl Cyanides and Chalcones作者:Mohammad M. Al-Arab、Bader S. Ghanem、Marilyn M. OlmsteadDOI:10.1055/s-1992-26289日期:——The synthesis of a number of highly substituted cyclohexane derivatives has been accomplished in a single step reaction of benzyl cyanides and chalcones (1:2) using sodium ethoxide in anhydrous diethyl ether at room temperature to give 3-aroyl-1,2,4, 6-tetraaryl-4-hydroxycyclohexanecarbonitriles. An unambiguous structural assignment was achieved from the analytical and infrared, 1H NMR and 13C NMR spectroscopic data as well as X-ray crystallography.
-
ANTI-INVASIVE COMPOUNDS申请人:Universiteit Gent公开号:US20150011620A1公开(公告)日:2015-01-08The present invention relates to the field of anti-invasive compounds and methods for predicting the anti-invasive activity of said compounds, as well as their use in the prevention and/or treatment of diseases associated with undesired cell invasion; in particular, this invention relates to the field of anti-invasive chalcone-like compounds.本发明涉及抗侵袭化合物领域,以及预测该类化合物抗侵袭活性的方法,以及它们在预防和/或治疗与不受欢迎的细胞侵袭相关的疾病中的用途;特别是,本发明涉及抗侵袭的类似香豆素化合物领域。
表征谱图
-
氢谱1HNMR
-
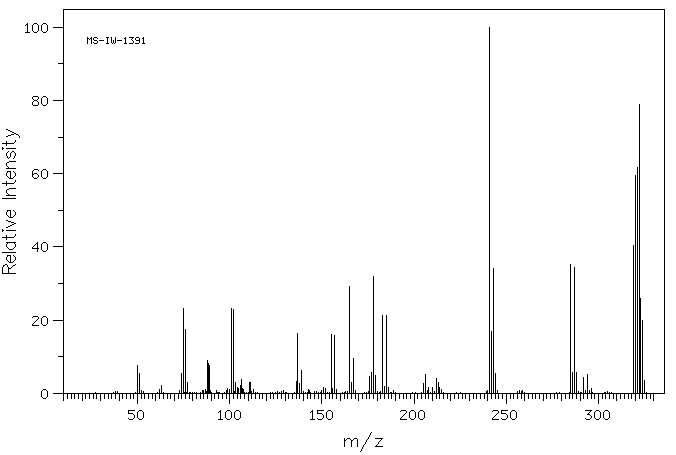
质谱MS
-
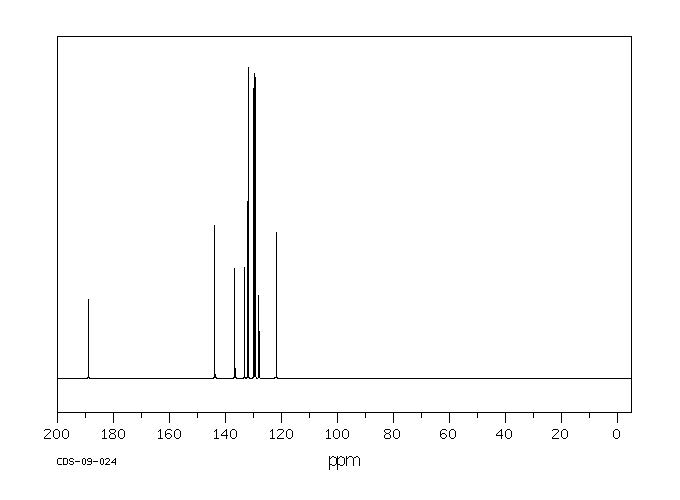
碳谱13CNMR
-
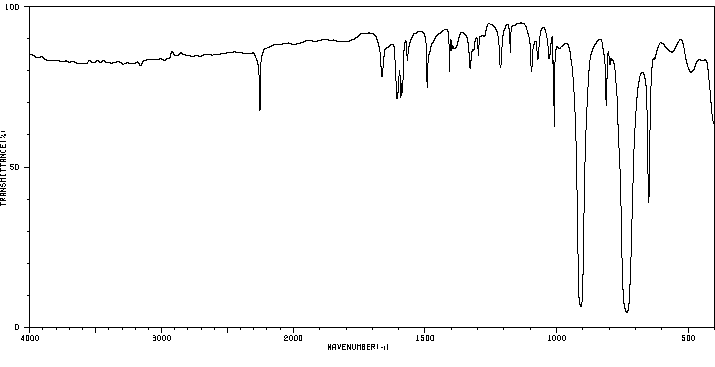
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2Z)-1,3-二苯基-2-丙烯-1-酮,2-丙烯-1-酮,1,3-二苯基-,(2Z)-
龙血素D
龙血素A
龙血素 B
黄色当归醇F
黄色当归醇B
黄腐醇; 黄腐酚
黄腐醇 D; 黄腐酚 D
黄腐酚B
黄腐酚
黄腐酚
黄卡瓦胡椒素 C
高紫柳查尔酮
阿普非农
阿司巴汀
阿伏苯宗
金鸡菊查耳酮
邻肉桂酰苯甲酸
达泊西汀杂质25
豆蔻明
补骨脂色烯查耳酮
补骨脂查耳酮
补骨脂呋喃查耳酮
补骨脂乙素
蜡菊亭; 4,2',4'-三羟基-6'-甲氧基查耳酮
苯酚,4-[3-(2-羟基苯基)-1-苯基丙基]-2-(3-苯基丙基)-
苯磺酰胺,N-[4-[3-(3-羟基苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,N-[3-[3-(4-羟基-3-甲氧苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,4-甲氧基-N,N-二甲基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯化,4,5-二甲氧基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯,4-甲氧基-3-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯甲醇,4-甲氧基-a-[2-(4-甲氧苯基)乙烯基]-
苯甲酸-[4-(3-氧代-3-苯基-丙烯基)-苯胺]
苯甲酸,3-[3-(4-溴苯基)-1-羰基-2-丙烯基]-4-羟基-
苯甲酰(2-羟基苯酰)甲烷
苯甲腈,4-(1-羟基-3-羰基-3-苯基丙基)-
苯基[2-(1-萘基)乙烯基]甲酮
苯基-(三苯基-丙-2-炔基)-醚
苯基-(2-苯基-2,3-二氢-苯并噻唑-2-基)-甲酮
苯亚甲基苯乙酮
苯乙酰腈,a-(1-氨基-2-苯基亚乙基)-
苯丙酸,a-苯甲酰-b-羰基-,苯基(苯基亚甲基)酰肼
苯,1-(2,2-二甲基-3-苯基丙基)-2-甲基-
苏木查耳酮
苄桂哌酯
苄基(4-氯-2-(3-氧代-1,3-二苯基丙基)苯基)氨基甲酸酯
芦荟提取物
腈苯唑
胀果甘草宁C
聚磷酸根皮酚