棕榈酰苯胺 | 6832-98-0
中文名称
棕榈酰苯胺
中文别名
N-苯基棕榈酰胺
英文名称
palmitanilide
英文别名
N-phenylpalmitamide;N-phenylhexadecanamide
CAS
6832-98-0
化学式
C22H37NO
mdl
MFCD00059287
分子量
331.542
InChiKey
VENJCACPSJGPCM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:90 °C
-
沸点:284 °C / 17mmHg
-
密度:0.9770 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):8.7
-
重原子数:24
-
可旋转键数:15
-
环数:1.0
-
sp3杂化的碳原子比例:0.681
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2924299090
-
储存条件:室温
SDS
Section I.Chemical Product and Company Identification
Chemical Name Palmitanilide
Portland OR
Synonym Hexadecanamide, N-Phenyl- (9 CI)
Chemical Formula CH3(CH2)14CONHC6H5
CAS Number 6832-98-0
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
Palmitanilide 6832-98-0 Min. 98.0 (T) Not available. Not available.
Section III. Hazards Identification
Acute Health Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this
compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
Inhalation If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the
toxic material was ingested; the absence of such signs, however, is not conclusive. SEEK IMMEDIATE MEDICAL
ATTENTION in case of ingestion of a radioactive material.
Section V. Fire and Explosion Data
Not available.
Flammability May be combustible at high temperature. Auto-Ignition
Flammable Limits
Flash Points Not available.
Not available.
Combustion Products These products are toxic carbon oxides (CO, CO 2), nitrogen oxides (NO, NO2).
Fire Hazards
Not available.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
and Instructions LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
6832-98-0rram
Section VI. Accidental Release Measures
Spill Cleanup Use a shovel to put the material into a convenient waste disposal container. Finish cleaning the spill by rinsing any
Instructions contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
Handling and Storage Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the container and store in a dry, cool
place. Avoid excessive heat and light. Do not breathe dust.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection Splash goggles. Lab coat. Dust respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult
a specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solid. (Powder.) Solubility
Physical state @ 20°C Not available.
Not available.
Specific Gravity
Molecular Weight 331.54 Partition Coefficient Not available.
Boiling Point
Not available. Vapor Pressure Not applicable.
Melting Point 90°C (194°F) Vapor Density Not available.
Refractive Index Not available. Volatility Not available.
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
RTECS Number Not available.
Eye Contact. Ingestion. inhalation.
Routes of Exposure
Toxicity Data Not available.
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Acute Toxic Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this
compound.
Continued on Next Page
Palmitanilide
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissove or mix material with a
Waste Disposal
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and locl regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
PIN Number Not applicable.
Proper Shipping Name
Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not available.
(Canada)
EINECS Number (EEC) Not available.
EEC Risk Statements Not available.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-hexadecylaniline 4439-42-3 C22H39N 317.558
反应信息
-
作为反应物:描述:参考文献:名称:Malki, Fatiha; Touati, Abdelkader; Rahal, Said, Asian Journal of Chemistry, 2011, vol. 23, # 3, p. 961 - 967摘要:DOI:
-
作为产物:参考文献:名称:十二烷基硫酸钠与苯磺酸盐基双子表面活性剂的合成,表征和混合胶束化研究摘要:在这里,我们显示了阴离子苯磺酸盐(即4,4'-(16,25-dioxo-15,17,24,26-四氮杂-六三aco烷15,26-二基)钠)二苯磺酸盐之间的混合胶束形成[BSC14- C6-14CSB]和4,4'-(18,27-dioxo-17,19,26,28-四氮杂-四contane15,26-二基)二苯磺酸钠[BSC16-C6-16CSB])与常规的阴离子表面活性剂(十二烷基钠)硫酸盐[SDS])通过电导和荧光法测定。在不同温度下,在一定范围内的SDS摩尔分数下进行电导率测量,以研究混合的胶束化和热力学参数,而在SDS的整个摩尔分数范围内进行荧光测量,以观察聚集和微极性。电导研究证实了在所有温度下SDS与[BSC14-C6-14CSB]和[BSC16-C6-16CSB]在所有摩尔分数中的协同作用。采用Rubinghs正解理论(RST)评估胶束摩尔分数,X 1,是混合胶束系统和吉布斯过量自由能(GDOI:10.1016/j.molliq.2019.04.057
文献信息
-
Chelating Bis(1,2,3‐triazol‐5‐ylidene) Rhodium Complexes: Versatile Catalysts for Hydrosilylation Reactions作者:Thanh V. Q. Nguyen、Woo‐Jin Yoo、Shū KobayashiDOI:10.1002/adsc.201500875日期:2016.2.4reactions. However, the substrates were mostly limited to reactive carbonyl compounds (aldehydes and ketones) or carbon‐carbon multiple bonds. Here, we describe the application of newly‐developed chelating bis(tzNHC)‐rhodium complexes (tz=1,2,3‐triazol‐5‐ylidene) for several reductive transformations. With these catalysts, the formal reductive methylation of amines using carbon dioxide, the hydrosilylation
-
CH Oxygenation and<i>N</i>-Trifluoroacylation of Arylamines Under Metal-Free Conditions: A Convenient Approach to 2-Aminophenols and<i>N</i>-Trifluoroacyl-<i>ortho</i>-aminophenols作者:Vunnam Venkateswarlu、K. A. Aravinda Kumar、Shilpi Balgotra、G. Lakshma Reddy、M. Srinivas、Ram A. Vishwakarma、Sanghapal D. SawantDOI:10.1002/chem.201402411日期:2014.5.26Direct ortho‐hydroxylation through CH oxygenation and N‐trifluoroacylation of anilines was achieved in a single step under metal‐free conditions by using a combination of TFA and oxone. The method allowed the formation of functionalised amino phenolic compounds such as ortho‐hydroxy‐N‐trifluoroacetanilides in good yields with broad substrate scope.
-
PROCESS FOR ALKENYLATING CARBOXAMIDES申请人:Staffel Wolfgang公开号:US20090131657A1公开(公告)日:2009-05-21The present invention relates to a process for preparing N-(1-alkenyl)carboxamides of the formula I, which comprises reacting a carboxamide of the formula II with an alkyne of the formula III in the presence of a catalyst selected from among carbonyl complexes, halides and oxides of rhenium, manganese, tungsten, molybdenum, chromium and iron.
-
HIGH CHEMOSELECTIVE SYNTHESIS OF CARBOXAMIDES BY USING<i>SYN</i>-PHENYLPYRIDYL-<i>O</i>-ACYL OXIMES(PPKO)
-
Amidation of Carboxylic Acids with Amines by Nb<sub>2</sub>O<sub>5</sub>as a Reusable Lewis Acid Catalyst作者:Md. A. Ali、S. M. A. H. Siddiki、Wataru Onodera、Kenichi Kon、Ken-ichi ShimizuDOI:10.1002/cctc.201500672日期:2015.11Among 28 types of heterogeneous and homogenous catalysts tested, Nb2O5 shows the highest yield for direct amidation of n‐dodecanoic acid with a less reactive amine (aniline). The catalytic amidation by Nb2O5 is applicable to a wide range of carboxylic acids and amines with various functional groups, and the catalyst is reusable. A comparison of the results of the catalytic study and an infrared study在测试的28种非均相和均相催化剂中,Nb 2 O 5对正十二烷酸与反应性较小的胺(苯胺)的直接酰胺化反应显示出最高的收率。由Nb 2 O 5催化的酰胺化适用于具有各种官能团的多种羧酸和胺,并且该催化剂是可重复使用的。催化研究和红外研究吸附在催化剂上的乙酸的结果比较表明,Nb 2 O 5上的路易斯酸位点活化了羧酸的羰基,这是Nb的高活性的原因。2 ø 5催化剂。动力学研究表明,Nb 2 O 5上的路易斯酸位比常规路易斯酸性氧化物(Al 2 O 3,TiO 2)具有更高的耐水性。与最新的酰胺化均相路易斯酸催化剂(ZrCl 4)相比,溶液中碱性添加剂对Nb 2 O 5的负面影响较小,这表明Nb 2 O 5对碱的耐受性更高。路易斯酸催化剂比均相路易斯酸催化剂。
表征谱图
-
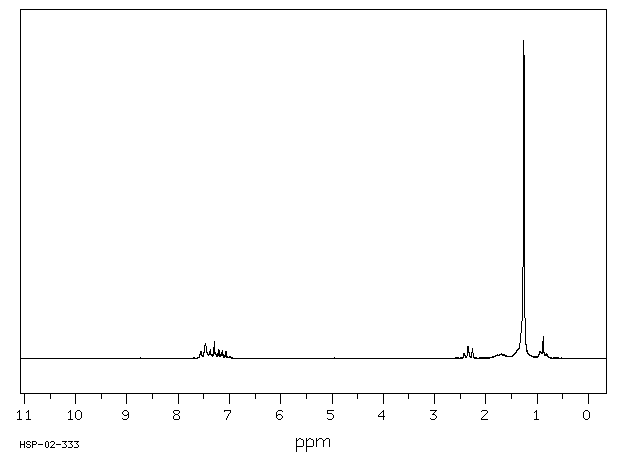
氢谱1HNMR
-
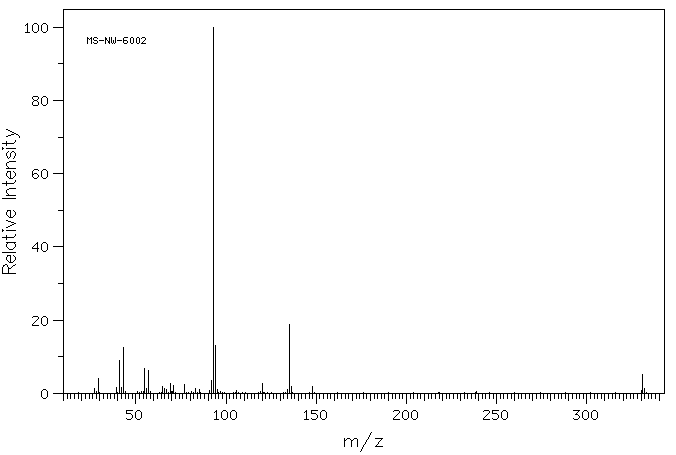
质谱MS
-
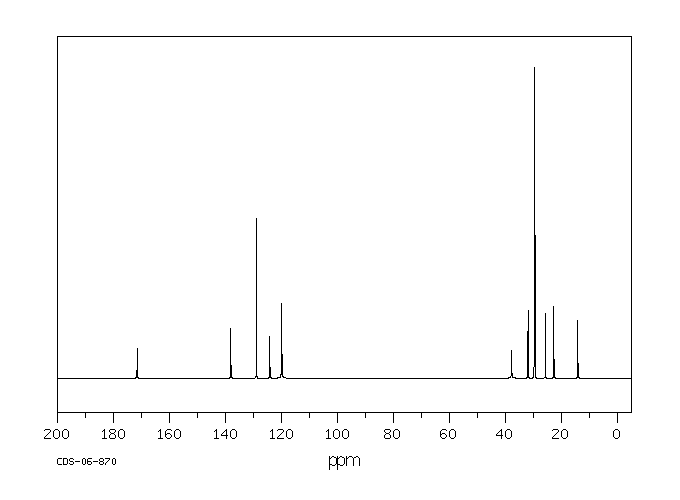
碳谱13CNMR
-
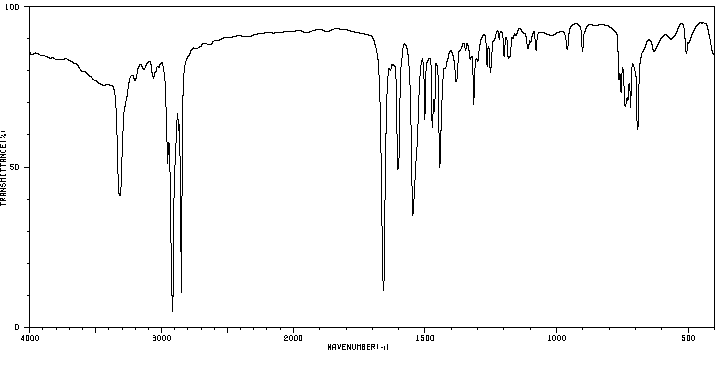
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫