1-(对甲苯基)-1-环戊烷羧酸 | 80789-75-9
中文名称
1-(对甲苯基)-1-环戊烷羧酸
中文别名
1-(对甲苯基)-1-环戊羧酸
英文名称
1-(4-methylphenyl)cyclopentane-1-carboxylic acid
英文别名
1-(p-Methylphenyl)cyclopentanecarboxylic acid;1-(p-methyl)cyclopentyl-1-carboxylic acid;1-(4-methylphenyl)cyclopentane carboxylic acid;1-(4-methylphenyl)cyclopentanecarboxylic acid;1-p-tolyl-cyclopentanecarboxylic acid
CAS
80789-75-9
化学式
C13H16O2
mdl
MFCD00044672
分子量
204.269
InChiKey
YKDWTRWSHHGVII-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:181-184 °C
-
沸点:302.76°C (rough estimate)
-
密度:1.0404 (rough estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.461
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
海关编码:29163900
-
WGK Germany:3
-
危险性防范说明:P261,P301+P312,P302+P352,P304+P340,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:存放于阴凉干燥处。
SDS
| Name: | 1-(p-Tolyl)-1-cyclopentanecarboxylic acid 98% Material Safety Data Sheet |
| Synonym: | 1-(4-Methylphenyl)-1-cyclopentane carboxylic aci |
| CAS: | 80789-75-9 |
Synonym:1-(4-Methylphenyl)-1-cyclopentane carboxylic aci
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 80789-75-9 | 1-(p-Tolyl)-1-cyclopentanecarboxylic a | 98 | 279-556-3 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation. Use with adequate ventilation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 80789-75-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: beige grey
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 182.00 - 183.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: C13H16O2
Molecular Weight: 204.27
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 80789-75-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-(p-Tolyl)-1-cyclopentanecarboxylic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 80789-75-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 80789-75-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 80789-75-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 1-(4-methylphenyl)cyclopentanecarboxylate 159499-64-6 C14H18O2 218.296 —— 1-(4-methylphenyl)cyclopentane carboxylic acid t-butyl ester 197659-52-2 C17H24O2 260.376 —— <1-(p-Methylphenyl)cyclopentyl>carbinol 162781-03-5 C13H18O 190.285 1-对甲苯环戊烷羰酰氯 1-p-Tolyl-cyclopentanecarbonyl chloride 82278-33-9 C13H15ClO 222.715 —— 6-Methylspiro[1-benzofuran-3,1'-cyclopentane]-2-one 1418027-95-8 C13H14O2 202.253
反应信息
-
作为反应物:描述:1-(对甲苯基)-1-环戊烷羧酸 在 氯化亚砜 、 三乙胺 作用下, 以 苯 为溶剂, 反应 16.0h, 生成 2-(pyrrolidin-1-yl)ethyl 1-(p-tolyl)cyclopentanecarboxylate hydrochloride参考文献:名称:Synthesis and Biological Activity of Arylcyclopentane-1-carboxylic Acids Aminoesters摘要:1-Arylcyclopentane-1-carboxylic acids were synthesized by alkaline hydrolysis of the corresponding nitriles. Reactions of the acid chlorides with N,N-dialkylaminoalkyl-and hetarylalkylalkanols provided new aminoester derivatives of 1-arylcyclopentane-1-carboxylic acids. Sympatholytic and adrenolytic activity of the obtained amino esters hydrochlorides was studied.DOI:10.1134/s107036321905027x
-
作为产物:描述:1-(4-甲基苯基)-1-氰基环戊烷 在 potassium hydroxide 作用下, 以 乙二醇 为溶剂, 反应 9.0h, 以90%的产率得到1-(对甲苯基)-1-环戊烷羧酸参考文献:名称:Synthesis and Biological Activity of Arylcyclopentane-1-carboxylic Acids Aminoesters摘要:1-Arylcyclopentane-1-carboxylic acids were synthesized by alkaline hydrolysis of the corresponding nitriles. Reactions of the acid chlorides with N,N-dialkylaminoalkyl-and hetarylalkylalkanols provided new aminoester derivatives of 1-arylcyclopentane-1-carboxylic acids. Sympatholytic and adrenolytic activity of the obtained amino esters hydrochlorides was studied.DOI:10.1134/s107036321905027x
文献信息
-
[EN] METHOD OF PREPARATION OF NOVEL NUCLEOSIDE ANALOGS AND USES<br/>[FR] PROCEDE POUR PREPARER DE NOUVEAUX ANALOGUES DE NUCLEOSIDE, ET LEURS UTILISATIONS申请人:AUSPEX PHARMACEUTICALS INC公开号:WO2005049582A1公开(公告)日:2005-06-02Processes for the preparation of racemic and optically active nucleoside analogs of formula (A) are described. These compounds are useful as anti-infective agents, antisense therapeutic agents and hybridization assay probes.描述了制备式(A)的外消旋和光学活性核苷类似物的过程。这些化合物可用作抗感染剂、反义治疗剂和杂交分析探针。
-
CXCR4 chemokine receptor binding comounds申请人:——公开号:US20040209921A1公开(公告)日:2004-10-21The present invention relates to compounds that bind to chemokine receptors, and having the formula 1 wherein each A, X, Y, R 1 , R 2 and R 3 are substituents. The present invention also relates to methods of using such compounds, such as in treating HIV infection and inflammatory conditions such as rheumatoid arthritis. Furthermore, the present invention relates to methods to elevate progenitor and stem cell counts, as well as methods to elevate white blood cell counts, using such compounds.本发明涉及与趋化因子受体结合的化合物,其具有式1的结构,其中A、X、Y、R1、R2和R3均为取代基。本发明还涉及使用这类化合物的方法,例如在治疗HIV感染和炎症性疾病如类风湿性关节炎中的应用。此外,本发明还涉及使用这类化合物来提高祖细胞和干细胞计数的方法,以及提高白细胞计数的方法。
-
[EN] COMPOUNDS HAVING MUSCARINIC RECEPTOR ANTAGONIST AND BETA2 ADRENERGIC RECEPTOR AGONIST ACTIVITY<br/>[FR] COMPOSÉS AYANT UNE ACTIVITÉ ANTAGONISTE D'UN RÉCEPTEUR MUSCARINIQUE ET AGONISTE D'UN RÉCEPTEUR BÊTA2 ADRÉNERGIQUE申请人:CHIESI FARMA SPA公开号:WO2012168359A1公开(公告)日:2012-12-13The present invention relates to compounds acting both as muscarinic receptor antagonists and beta2 adrenergic receptor agonists, to processes for their preparation, to compositions comprising them, to therapeutic uses and combinations with other pharmaceutical active ingredients.
-
Monoprotected Amino Acid (MPAA) Ligand Enabled C–H Alkynylation of Phenyl Acetic Acid作者:Yue-Jin Liu、Zheng-Xin Zhou、Di Xie、Xiao-Peng Luo、Hao Wang、Bin Liu、Ming-Hua ZengDOI:10.1021/acs.orglett.8b03182日期:2018.11.16A weakly carboxylate-directed palladium(II)-catalyzed ortho-C–H alkynylation of diverse phenylacetic acids promoted by monoprotected amino acid ligand enabled is reported. The reaction has a broad substrate scope including α-secondary, tertiary, and quaternary phenylacetic acids. Notably, the direct ortho-C–H alkynylation of α-quaternary phenylacetic acids and chiral α-tertiary phenylacetic acids was
-
Compounds useful as phosphotyrosine mimics申请人:Boehringer Ingelheim Pharmaceuticals, Inc.公开号:US06156784A1公开(公告)日:2000-12-05Disclosed are compositions containing compounds of the formula (I) below wherein A,B,C,G,Q and R are defined herein. The compounds are useful as phosphotyrosine mimics that, when incorporated into an appropriate molecular structure, inhibit the binding of tyrosine kinase-dependent regulatory proteins to their native phosphotyrosine-containing ligands or receptors. Also disclosed are methods for preparing the compounds of the formula (I). ##STR1##
表征谱图
-
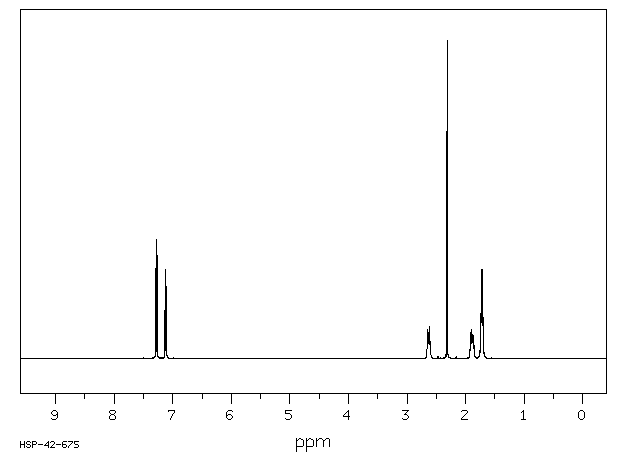
氢谱1HNMR
-
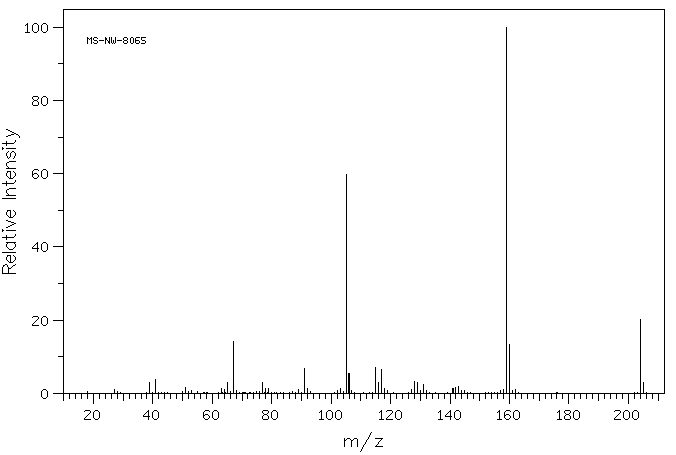
质谱MS
-
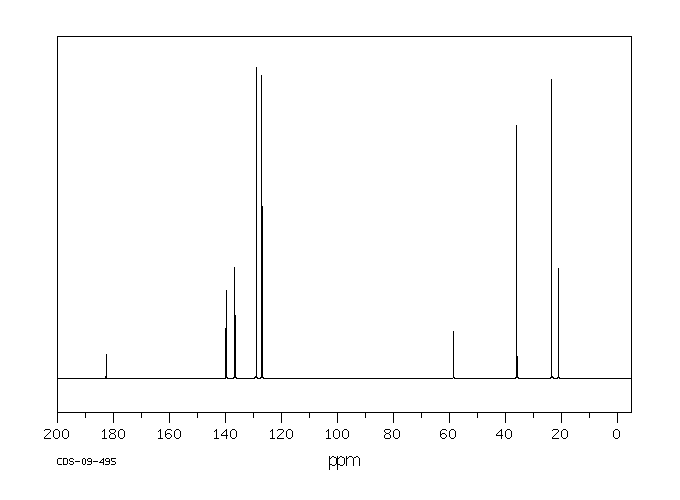
碳谱13CNMR
-
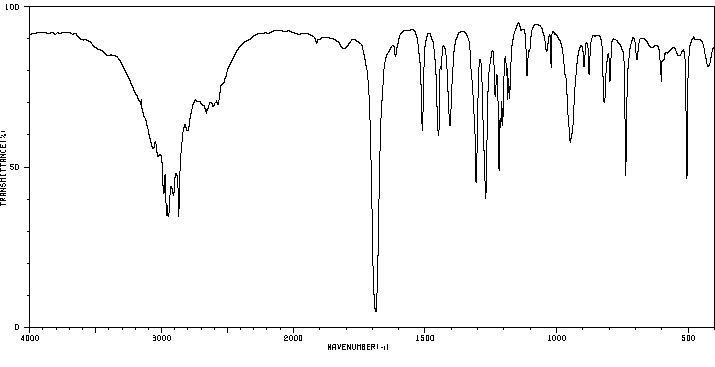
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫