1-(甲氧基甲基)-4-(三氟甲基)苯 | 155820-05-6
中文名称
1-(甲氧基甲基)-4-(三氟甲基)苯
中文别名
——
英文名称
4-(trifluoromethyl)benzyl methyl ether
英文别名
1-(methoxymethyl)-4-(trifluoromethyl)benzene
CAS
155820-05-6
化学式
C9H9F3O
mdl
MFCD11504959
分子量
190.165
InChiKey
VSEBVHPRKPUKQD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:169.1±35.0 °C(Predicted)
-
密度:1.172±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:4
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 1-(Methoxymethyl)-4-(trifluoromethyl)benzene
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-(Methoxymethyl)-4-(trifluoromethyl)benzene
CAS number: 155820-05-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H9F3O
Molecular weight: 190.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen fluoride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 1-(Methoxymethyl)-4-(trifluoromethyl)benzene
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-(Methoxymethyl)-4-(trifluoromethyl)benzene
CAS number: 155820-05-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H9F3O
Molecular weight: 190.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen fluoride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-(三氟甲基)苄醇 4-(trifluoromethyl)benzylic alcohol 349-95-1 C8H7F3O 176.138 —— 4-(trifluoromethyl)benzyl acetate 89751-95-1 C10H9F3O2 218.175 —— 4-(trifluoromethyl)benzyl pivalate —— C13H15F3O2 260.256 4-(三氟甲基)苄基氯 4-trifluoromethylbenzyl chloride 939-99-1 C8H6ClF3 194.584 对三氟甲基苯甲醛 4-Trifluoromethylbenzaldehyde 455-19-6 C8H5F3O 174.122 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(三氟甲基)苯甲酸甲酯 methyl 4-(trifluoromethyl)benzoate 2967-66-0 C9H7F3O2 204.149 对三氟甲基苯甲醛 4-Trifluoromethylbenzaldehyde 455-19-6 C8H5F3O 174.122
反应信息
-
作为反应物:描述:参考文献:名称:吸附效应对CH 3 CN中苄基衍生物的TiO 2敏化光氧化速率的影响摘要:在充气和/或脱气的CH 3 CN中,在Ag 2 SO 4的存在下,进行了一系列不同环取代的苄基衍生物(ArCHROR')在TiO 2敏化的光氧化反应中的竞争动力学实验。根据log k rel vs E p图,假设电子抽象位点的变化是在环上从给电子基团到吸电子基团,从芳族部分到苄基甲基醚的OCH 3基团发生的由于该基团优先吸附在TiO 2上关于芳环。在苄基三甲基硅烷中未观察到该现象,因为由C-Si键和芳族π系统重叠产生的轨道是唯一的吸附位点。α-甲基(在苄醇中)或叔丁基(在苄基叔丁基醚中)的空间位阻会降低吸附程度,但不会影响图的形状,因为OR'应该仍然是首选吸附基团。这也提供了有关悬浮在CH 3 CN中的TiO 2的表面结构的有用信息。版权所有©2003 John Wiley&Sons,Ltd.DOI:10.1002/poc.583
-
作为产物:描述:对三氟甲基苯甲醛 在 甲醇 、 sodium tetrahydroborate 、 sodium hydride 作用下, 以 四氢呋喃 、 paraffin oil 为溶剂, 反应 1.0h, 生成 1-(甲氧基甲基)-4-(三氟甲基)苯参考文献:名称:氧铵介导的烯丙基硅烷-醚偶联反应摘要:公开了使用烯丙基硅烷作为亲核伙伴的烯丙基和苄基醚的氧铵介导的α-C(sp 3)烯丙基化反应。这种新的形成C-H键的C-C键可允许以中等到良好的产率获得相应的偶联产物。DOI:10.1002/ejoc.202100026
文献信息
-
Enzymatic assembly of carbon–carbon bonds via iron-catalysed sp3 C–H functionalization作者:Ruijie K. Zhang、Kai Chen、Xiongyi Huang、Lena Wohlschlager、Hans Renata、Frances H. ArnoldDOI:10.1038/s41586-018-0808-5日期:2019.1produced in bacteria, where they can be tuned by directed evolution for activity and selectivity. That these proteins activate iron, the most abundant transition metal, to perform this chemistry provides a desirable alternative to noble-metal catalysts, which have dominated the field of C–H functionalization1,2. The laboratory-evolved enzymes functionalize diverse substrates containing benzylic, allylic尽管在有机分子中含量丰富,但碳氢 (C-H) 键通常被认为是非反应性的,不可用于化学操作。C-H 功能化技术的最新进展已经开始改变这种逻辑,同时强调了与 sp3 carbon1,2 选择性烷基化相关的重要性和挑战。在这里,我们描述了通过卡宾 C-H 插入对 sp3 C-H 键进行对映、区域和化学选择性分子间烷基化的铁基催化剂。催化剂源自细胞色素 P450 酶,其中天然半胱氨酸轴向配体已被丝氨酸(细胞色素 P411)取代,完全遗传编码并在细菌中产生,可通过定向进化调节活性和选择性。这些蛋白质激活铁,最丰富的过渡金属,进行这种化学反应为贵金属催化剂提供了一种理想的替代品,贵金属催化剂在 C-H 功能化领域占主导地位 1,2。实验室开发的酶以高周转率和优异的选择性功能化含有苄基、烯丙基或 α-氨基 C-H 键的多种底物。此外,它们使开发几种天然产品的简洁路线成为可能。使用这些酶的天然铁 - 血红素辅因子介导
-
8-AZABICYCLO[3.2.1]OCTANE-8-CARBOXAMIDE DERIVATIVE申请人:Horiuchi Yoshihiro公开号:US20120225876A1公开(公告)日:2012-09-06Disclosed is a compound represented by formula (1) or a pharmacologically acceptable salt thereof (In the formula, A represents a group that is represented by formula (A-1); R 1a and R 1b may be the same or different and each independently represents a C 1-6 alkyl group which may be substituted by one to three halogen atoms; m and n each independently represents an integer of 0-5; X 1 represents a hydroxyl group or an aminocarbonyl group; Z 1 represents a single bond or the like; and R 2 represents an optionally substituted C 1-6 alkyl group, an optionally substituted C 6-10 aryl group or the like.)公开了一种由公式(1)表示的化合物或其药理可接受的盐(在公式中,A代表由公式(A-1)表示的基团;R1a和R1b可以相同或不同,每个独立地表示一个可以由一个到三个卤素原子取代的C1-6烷基;m和n各自独立地表示0-5之间的整数;X1代表羟基或氨基甲酰基;Z1代表单键等;R2代表一个可选地取代的C1-6烷基,一个可选地取代的C6-10芳基等)。
-
Correlation of the rates of solvolysis of (arylmethyl)methylphenylsulfonium ions †作者:Dennis N. Kevill、Norsaadah HJ IsmailDOI:10.1039/a803859g日期:——The specific rates of solvolysis of the benzylmethylphenylsulfonium ion (prepared as the trifluoromethanesulfonate salt) and five benzylic ring-substituted derivatives can be satisfactorily correlated using NT solvent nucleophilicity values. Addition of a secondary term, governed by the aromatic ring parameter (I), shows the sensitivities towards changes in this parameter to fall and those towards所述benzylmethylphenylsulfonium离子(如三氟甲磺酸盐盐制备)和5苄基环上取代的衍生物的溶剂分解的特定速率可以使用良好地相关Ñ Ť溶剂的亲核性值。加入第二术语的,由芳环的参数(约束我),示出了向在该参数中的变化的灵敏度下降,这些朝向变化Ñ Ť随取代基的吸电子能力上升。具有吸电子取代基的哈米特ρ值(基于σ +值)从95%丙酮中的–0.9到97%2,2,2-三氟乙醇中的–1.8不等。将这些Grunwald-Winstein和Hammett分析与以前报道的,使用基本相同的溶剂和取代基进行的芳基甲基对甲苯磺酸盐溶剂化方法进行了比较。
-
Broad-Scope Rh-Catalyzed Inverse-Sonogashira Reaction Directed by Weakly Coordinating Groups作者:Eric Tan、Ophélie Quinonero、M. Elena de Orbe、Antonio M. EchavarrenDOI:10.1021/acscatal.7b04395日期:2018.3.2We report the alkynylation of C(sp2)-H bonds with bromoalkynes (inverse-Sonogashira reaction) directed by synthetically useful ester, ketone, and ether groups under rhodium catalysis. Other less common directing groups such as amine, thioether, sulfoxide, sulfone, phenol ester, and carbamate are also suitable directing groups. Mechanistic studies indicate that the reaction proceeds by a turnover-limiting
-
[EN] PURINE AND PYRIMIDINE NUCLEOTIDES AS ECTO-5'-NUCLEOTIDASE INHIBITORS<br/>[FR] NUCLÉOTIDES DE PURINE ET DE PYRIMIDINE EN TANT QU'INHIBITEURS DE L'ECTO-5'-NUCLÉOTIDASE申请人:US HEALTH公开号:WO2020037275A1公开(公告)日:2020-02-20Disclosed is a compound of formula (I), wherein Q, U, T, A, a, b, c, and n are as defined herein. Also disclosed are methods of inhibiting ecto‑5'‑nucleotidase, inhibiting suppression of an antitumor immune response, inhibiting tumor growth of a cancerous tumor, inhibiting metastasis of cancer in a mammal afflicted with cancer, synergistically enhancing a response of a mammal afflicted with cancer undergoing treatment with an immunotherapeutic anti‑cancer agent, potentiating an activity of an inhibitor of nicotinamide phosphoribosyltransferase in a mammal undergoing treatment of a mammal with the inhibitor, and treating preeclampsia in a mammal in need thereof, comprising administering to an animal an effective amount of a compound of formula (I).
表征谱图
-
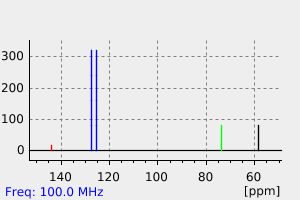
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫