1-氨基-5-苯甲酰胺基蒽醌 | 117-06-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:26
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:89.3
-
氢给体数:2
-
氢受体数:4
安全信息
-
海关编码:2924299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-(5-氯-9,10-二氧代蒽-1-基)苯甲酰胺 1-Chloro-5-benzoylaminoanthraquinone 117-05-5 C21H12ClNO3 361.784 —— 1-benzoylamino-5-nitro-anthraquinone 62089-79-6 C21H12N2O5 372.337 1,5-二氨基蒽醌 1,5-diaminoanthraquinone 129-44-2 C14H10N2O2 238.246 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N,N'-二[5-(苯甲酰基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]间苯二甲酰胺 N,N'-bis-(5-benzoylamino-9,10-dioxo-9,10-dihydro-[1]anthryl)-isophthalamide 6370-78-1 C50H30N4O8 814.81 还原黄12 N,N'-bis-(5-benzoylamino-9,10-dioxo-9,10-dihydro-[1]anthryl)-oxalamide 6370-75-8 C44H26N4O8 738.712 N-[5-[[5-(苯甲酰基氨基)-9,10-二氧代蒽-1-基]氨基]-9,10-二氧代蒽-1-基]苯甲酰胺 5,5'-bis-benzoylamino-1,1'-imino-di-anthraquinone 129-28-2 C42H25N3O6 667.677 N-[4-[[5-(苯甲酰基氨基)-9,10-二氧代蒽-1-基]氨基]-9,10-二氧代蒽-1-基]苯甲酰胺 4,5'-bis-benzoylamino-1,1'-imino-di-anthraquinone 128-89-2 C42H25N3O6 667.677 —— 1-S,S-dimethyl-N-(5-benzamidoanthraquinon-1-yl)sulfoximide 85193-02-8 C23H18N2O4S 418.473 氨基-9,10-蒽二酮 1-amino-9,10-anthracenedione 82-45-1 C14H9NO2 223.231 1,1'-亚氨基二蒽醌 1,1'-dianthrimide 82-22-4 C28H15NO4 429.431 —— 1-S,S-dimethyl N-(5-aminoanthraquinon-1-yl)sulfoximide 85193-20-0 C16H14N2O3S 314.365
反应信息
-
作为反应物:描述:参考文献:名称:Wittenberger, Melliand Textilberichte, 1949, vol. 30, p. 159摘要:DOI:
-
作为产物:参考文献:名称:Acylamino-anthraquinones and process of producing the same摘要:公开号:US01939218A1
文献信息
-
Zur Kenntnis der Dinitro-anthrachinone作者:Eugen HeftiDOI:10.1002/hlca.19310140620日期:1931.12.1Bei der Dinitrierung des Anthrachinons nach den bekannten gebräuchlichen Methoden wurden rein erhalten: ca. 40% 1,5-Dinitro-anthrachinon vom Smp. 384,5–385° 37% 1,8-Dinitro-anthrachinon vom Smp. 311–312°, ferner 12–15% 1,6-Dinitro-anthrachinon vom Smp. 255–257°. Das 1,7-Dinitro-anthrachinon konnte nur unrein gewonnen werden, während die 2,6- und 2,7-Dinitro-anthrachinone überhaupt nicht isoliert wurden
-
Acetoacetylamino compounds and a process of making same申请人:DURAND &公开号:US02776983A1公开(公告)日:1957-01-08
The invention comprises the N-acetoacetyl derivative of 1-chloro-2-aminoanthraquinone, and the preparation thereof and of other acetoacetylaminoanthraquinones by heating aminoanthraquinones with diketene in the presence of 90-100 per cent (preferably glacial) acetic acid at 40-80 DEG C. (preferably 60-70 DEG C.). Examples describe the acetoacetylation of 1- and 2-aminoanthraquinone, 1- and 3-chloro-2-aminoanthraquinone and 1-amino - 5 - benzoylaminoanthraquinone, and the di-acetoacetylation of 1 : 5-diaminoanthraquinone. Additional starting materials specified are 1-amino-4-benzoylaminoanthraquinone, 1 - amino - 4 - phenylaminoanthraquinone and 1 : 4 - diaminoanthraquinone. Specification 429,982 is referred to.ALSO:The invention comprises N - acetoacetyl derivatives of 4-benzoylaminodiphenylamine, carbazole, 1-aminobenzene-4-sulphonic acid 21-thiazolylamide, and naphthosultam, and the preparation thereof and of other acetoacetyl-amino compounds by heating a primary or secondary aromatic amine, or a heterocyclic compound containing a secondary nitrogen atom in the heterocyclic nucleus, with diketene in the presence of 90-100 per cent (preferably glacial) acetic acid at 40-80 DEG C. (preferably 60-70 DEG C.). Examples describe the acetoacetylation of the four compounds specified above and of 1-aminobenzene-4-sulphonic acid morpholide and diphenylamine. Additional starting materials specified include benzidine naphthoquinone, 1-aminobenzene-4-sulphonic acid p-phenylanilide and dimethylamide, 4-aminodiphenylsulphone, and N-phenyl-1-and-2-naphthylamine. Specification 429,982 is referred to.
本发明涉及1-氯-2-氨基蒽醌的N-乙酰乙酰衍生物,以及通过在90-100%(优选为冰醋酸)乙酸存在下,在40-80℃(优选为60-70℃)下加热氨基蒽醌和二酮烯制备其他乙酰乙酰氨基蒽醌和1-和2-氨基蒽醌、1-和3-氯-2-氨基蒽醌、1-氨基-5-苯甲酰氨基蒽醌的例子描述,并且对1:5-二氨基蒽醌进行了双乙酰乙酰化。此外,指定了其他起始材料,包括1-氨基-4-苯甲酰氨基蒽醌、1-氨基-4-苯基氨基蒽醌和1:4-二氨基蒽醌。参考专利规格书429,982。 此外,本发明还涉及4-苯甲酰氨基二苯胺、咔唑、1-氨基苯并-4-磺酸21-噻唑酰胺和萘磺酰胺的N-乙酰乙酰衍生物,以及通过在90-100%(优选为冰醋酸)乙酸存在下,在40-80℃(优选为60-70℃)下加热含有二级氮原子的芳香胺或杂环化合物,制备其他乙酰乙酰氨基化合物。例子描述了上述四种化合物和1-氨基苯并-4-磺酸吗啉酰和二苯胺的乙酰乙酰化,指定的其他起始材料包括苯二胺萘醌、1-氨基苯并-4-磺酸对苯基苯胺酰和二甲基酰胺、4-氨基二苯磺酮和N-苯基-1-和2-萘胺。参考专利规格书429,982。 -
PREPARATION METHOD OF ORIGINAL DYE OF VAT BROWN R申请人:Xiang Dezhi公开号:US20120296097A1公开(公告)日:2012-11-22A preparation method of original dye of Vat Brown R comprises the following steps: a. after acylation of 1,5-diaminoanthraquinone, 1-amino-5-benzamidoanthraquinone was prepared by acidic hydrolysis; b. 1-benzamido-4-bromoanthraquinone was obtained from 1-aminoanthraquinone by acylation and bromination; c. a condensate of Vat Brown R was obtained by condensation reaction of 1-amino-5-benzamidoanthraquinone and 1-benzamido-4-bromoanthraquinone; d. the original dye of Vat Brown R was obtained from the condensate of Vat Brown R by ring closing reaction and oxidation reaction. The method omits one oxidation step, economizes significant amount of oxidizing agent, and reduces significant amount of waste water, so it is very beneficial to environment protection; and the method also exhibited the advantages of highly increasing product yield and reducing the costs of raw materials to an extent of more than 30%.
-
Process for the preparation of anthraquinone imides申请人:Ciba-Geigy AG公开号:US04659831A1公开(公告)日:1987-04-21The invention relates to a process for the preparation of anthraquinone imides by condensing vattable anthraquinone compounds which contain at least one primary amino group with aromatic halogen compounds, in an organic solvent and at elevated temperature in the presence of a base and a copper catalyst. The process comprises first carrying out the condensation until a reaction of 60-95% has taken place, and then subjecting the reaction mixture, without isolating the reaction product, to an aftertreatment at a temperature which is 5.degree. to 60.degree. C. above the initial temperature of the condensation. This process permits the anthraquinone imides to be obtained in increased yield and additionally provides products with improved qualities. The intermediates so obtained can be further processed to vat dyes.
-
Organic dyes containing silane groups and process for preparing same申请人:Montedison S.p.A.公开号:US04609404A1公开(公告)日:1986-09-02This invention relates to organic dyes containing silane groups; composite pigments obtainable by grafting said dyes onto an inorganic substrate; and, the respective processes for preparing said dyes and composite pigments. The dyes have general formula: ##STR1## wherein R.sub.1 and R.sub.2, which may be equal or different, are selected from the group consisting of H, --NH.sub.2, --NHR wherein R is an alkyl group having from 1 to 4 carbon atoms or a phenyl group, --NHCOCH.sub.3, --NHCOC.sub.6 H.sub.5, --NO.sub.2, a halogen, --OH, --O--C.sub.6 H.sub.5 and --S--C.sub.6 H.sub.5 ; n is a number selected from 3, 4 and 5; W is selected from the group consisting of an alkyl group having from 1 to 4 carbon atoms and a phenyl group; Y is an alkoxy group having from 1 to 4 carbon atoms; q is a number selected from 0 and 1; X is 3 and m is a number selected from 0, 1, 2 and 3 when q is 0; x is 2 and m is a number selected from 0, 1 and 2 when q is 1. The composite pigments obtained from said dyes are employed in the painting products, air enamels and stoving enamels, in the pigmentation of plastic materials, in the inks and in the printing of fabrics.本发明涉及含有硅烷基的有机染料;通过将该染料嫁接到无机基质上获得的复合颜料;以及制备该染料和复合颜料的相应过程。该染料具有通式:##STR1## 其中R.sub.1和R.sub.2(可以相等或不同)选自包括H,--NH.sub.2,--NHR(其中R是具有1到4个碳原子的烷基或苯基),--NHCOCH.sub.3,--NHCOC.sub.6 H.sub.5,--NO.sub.2,卤素,--OH,--O--C.sub.6 H.sub.5和--S--C.sub.6 H.sub.5的群;n是选自3、4和5的数字;W选自具有1到4个碳原子的烷基和苯基的群;Y是具有1到4个碳原子的烷氧基;q是选自0和1的数字;当q为0时,X为3,m是选自0、1、2和3的数字;当q为1时,x为2,m是选自0、1和2的数字。从该染料获得的复合颜料用于涂料产品、空气漆和烘烤漆中、塑料材料的着色、油墨和织物印刷中。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
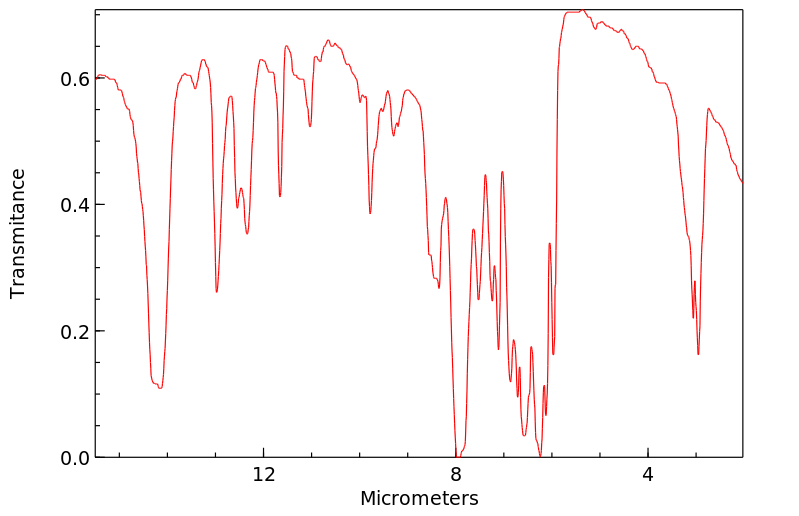
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息