1-甲基-4(1H)-吡啶酮 | 695-19-2
中文名称
1-甲基-4(1H)-吡啶酮
中文别名
——
英文名称
N-methyl-4-pyridone
英文别名
1-methyl-4-pyridone;N-Methyl-pyridon-(4);N-Methyl-4-pyridon;1-Methyl-4-pyridon;1-Methyl-pyridon-(4);1-methylpyridin-4(1H)-one;N-Methyl-γ-pyridon;1-methyl-4(1H)-pyridone;1-Methyl-4(1H)-pyridon;1-Methyl-4(1H)-pyridinone;1-methylpyridin-4-one
CAS
695-19-2
化学式
C6H7NO
mdl
——
分子量
109.128
InChiKey
OYPBUQASUUMJKG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:92-98°C (dec.)
-
沸点:204.59°C (rough estimate)
-
密度:1.1143 (rough estimate)
-
溶解度:氯仿(微溶)、甲醇(微溶)
计算性质
-
辛醇/水分配系数(LogP):-1.2
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:20.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2933399090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-羟基吡啶 pyridin-4-ol 626-64-2 C5H5NO 95.1008
反应信息
-
作为反应物:描述:1-甲基-4(1H)-吡啶酮 在 硫酸 、 三氧化硫 、 potassium nitrate 作用下, 反应 5.0h, 以52.1%的产率得到3,5-dinitro-1-methyl-4-pyridone参考文献:名称:Nucleophilic Reaction of Electron-deficient Pyridone Derivatives. II. Ring Transformation of 1-Substituted 3,5-Dinitro-4-pyridones with Sodio β-Keto Esters摘要:1-取代的3,5-二硝基-4-吡啶酮 [1-取代基:甲基 (1a)、2-吡啶基 (1b)、6-甲基-2-吡啶基 (1c) 和4-吡啶基 (1d)] 与二乙基钠-3-氧代戊二酸酯的反应生成1-取代的3,5-双(乙氧基羧基)-4-吡啶酮和钠-1,3-二硝基-2-丙烷。另一方面,4-吡啶酮 (1b、1c 和 1d) 与乙基钠乙酰乙酸酯的反应生成乙基4-羟基-3,5-二硝基苯甲酸酯及其氨基吡啶衍生物,而1a的反应则生成呋喹[3,2-b]吡啶衍生物。基于软酸和硬酸及碱的概念,建议提出β-酮酯阴离子对4-吡啶酮的2和6位或2和3位的亲核攻击为逐步发生,以解释反应路线的变化。DOI:10.1246/bcsj.53.2891
-
作为产物:描述:参考文献:名称:Haitinger; Lieben, Monatshefte fur Chemie, 1885, vol. 6, p. 315摘要:DOI:
文献信息
-
SILICON BASED DRUG CONJUGATES AND METHODS OF USING SAME申请人:BlinkBio, Inc.公开号:US20170202970A1公开(公告)日:2017-07-20Described herein are silicon based conjugates capable of delivering one or more payload moieties to a target cell or tissue. Contemplated conjugates may include a silicon-heteroatom core, one or more optional catalytic moieties, a targeting moiety that permits accumulation of the conjugate within a target cell or tissue, one or more payload moieties (e.g., a therapeutic agent or imaging agent), and two or more non-interfering moieties covalently bound to the silicon-heteroatom core.
-
[EN] MUSCARINIC ACETYLCHOLINE M1 RECEPTOR ANTAGONISTS<br/>[FR] ANTAGONISTES DES RÉCEPTEURS MUSCARINIQUES DE L'ACÉTYLCHOLINE M1申请人:PIPELINE THERAPEUTICS INC公开号:WO2021071843A1公开(公告)日:2021-04-15Provided herein, inter alia, are compounds which are useful as antagonists of the muscarinic acetylcholine receptor M1 (mAChR M1); synthetic methods for making the compounds; pharmaceutical compositions comprising the compounds; and methods of treating neurological and psychiatric disorders associated with muscarinic acetylcholine receptor dysfunction using the compounds and compositions.
-
[EN] 6-(5-HYDROXY-1H-PYRAZOL-1-YL)NICOTINAMIDE DERIVATIVES AND THEIR USE AS PHD INHIBITORS<br/>[FR] INHIBITEURS 6-(5-HYDROXY-1H-PYRAZOL-1-YL)NICOTINAMIDE DE PHD申请人:TAKEDA PHARMACEUTICAL公开号:WO2014160810A1公开(公告)日:2014-10-02The present invention provides compounds of formula (I) which are useful as inhibitors of PHD, pharmaceutical compositions thereof, methods for treatment of conditions associated with HIF, processes for making the compounds and intermediates thereof.本发明提供了公式(I)的化合物,这些化合物可用作PHD的抑制剂,以及与HIF相关疾病的治疗方法,制备这些化合物及其中间体的药物组合物。
-
Alkylation of Pyridone Derivatives By Nickel/Lewis Acid Catalysis作者:Ryuichi Tamura、Yuuya Yamada、Yoshiaki Nakao、Tamejiro HiyamaDOI:10.1002/anie.201200922日期:2012.6.4MAD as an additive: The [Ni(cod)2], (2,6‐tBu2‐4‐MeC6H2O)2AlMe (MAD), and N‐heterocyclic carbene (NHC) catalytic system effected a highly regioselective alkylation of pyridone derivatives (see scheme). Substituted pyridones and related heterocycles react with both terminal and internal alkenes to selectively give a range of nitrogen‐containing heterocycles with linear alkyl substituents.
-
Structural considerations for charge‐enhanced Brønsted acid catalysts作者:Curtis Payne、Steven R. KassDOI:10.1002/poc.4069日期:2020.8All three N‐methylated and N‐protonated hydroxypyridinium BArF4– salt isomers were synthesized and their hydrogen bond donating abilities were investigated. DFT and G4 theory computations along with IR spectroscopic measurements were found to be effective methods for predicting the catalytic activities of these O–H and N–H Brønsted acids. A UV‐vis titration approach for rapidly quantifying hydrogen
表征谱图
-
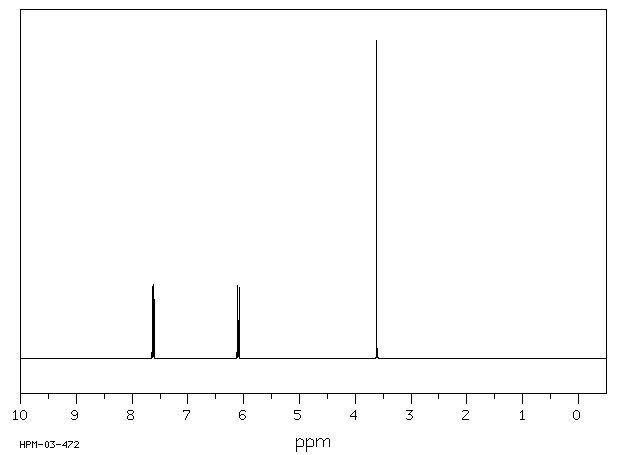
氢谱1HNMR
-
质谱MS
-
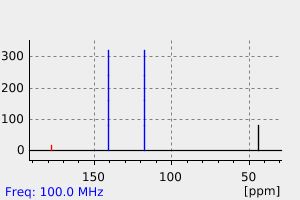
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-