1-硝基菲 | 17024-17-8
中文名称
1-硝基菲
中文别名
——
英文名称
1-nitrophenanthrene
英文别名
1-Nitro-phenanthren
CAS
17024-17-8
化学式
C14H9NO2
mdl
——
分子量
223.231
InChiKey
SGNWFFATVZVHNF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:73.5 °C
-
沸点:364.52°C (rough estimate)
-
密度:1.1814 (rough estimate)
-
保留指数:2115.2;366.1;353.4
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:17
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2904209090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Dewar; Mole, Journal of the Chemical Society, 1956, p. 2556摘要:DOI:
-
作为产物:描述:菲 在 Iron(III) nitrate nonahydrate 作用下, 反应 1.0h, 以94%的产率得到1-硝基菲参考文献:名称:机械力作用下实现硝酸盐硝化芳香族化合物 的方法摘要:本发明公开了机械力作用下实现硝酸盐硝化芳香族化合物的方法。本发明提供的一种芳香族硝基化合物的制备方法,包括如下步骤:在机械力作用下,芳香族化合物与金属硝酸盐或其水合物经硝化反应,得到所述芳香族硝基化合物;所述机械力为机械提供的能引起物质物理和/或化学性质变化的外力。所述机械力可为压缩、剪切、冲击、摩擦、拉伸、弯曲和振动中的任一种。本发明具有如下优点:无需使用任何溶剂,避免了废液产生;不需使用酸性物质,反应完成后处理简单,对设备无任何损伤;极高的转化率和选择性,可以应用于硝化常规芳香族化合物。公开号:CN108409575B
文献信息
-
유기 화합물 및 이를 포함하는 유기 전계 발광 소자申请人:DOOSAN CORPORATION 주식회사 두산(119987140410) Corp. No ▼ 110111-0013774BRN ▼107-81-00237公开号:KR20150070617A公开(公告)日:2015-06-25본 발명은 정공 주입 및 수송능, 발광능 등이 우수한 신규의 벤조피롤카바졸계 화합물 및 이를 하나 이상의 유기물층에 포함함으로써 발광효율, 구동전압, 수명 등의 특성이 향상된 유기 전계 발광 소자에 관한 것이다.
-
Sustainable chemical process for reduction of nitro compounds (R-NO2) or nitroso compounds (R-NO) containing sulphonic or carboxylic group into corresponding amino compounds (R-NH2) with inherent recycle of all acidic streams generated in synthesis申请人:Padia Bhadresh K.公开号:US20120203031A1公开(公告)日:2012-08-09The process of the present invention creates a sustainable and closed water loop allowing inherent recycles of all liquid streams generated in the process. The liquid streams generated during the process of the invention are inherently recycled completely, making the process of the present invention a zero liquid discharge process which is environmentally friendly and sustainable. This invention further relates to a sustainable chemical process of reduction of R—NO 2 or R—NO into corresponding R—NH 2 that produces environmentally friendly R—NH 2 in good yields and selectivity with large of mother liquor recycle. The process has a wide scope in that it can be applied to a number of molecules.
-
[EN] SUSTAINABLE CHEMICAL PROCESS FOR REDUCTION OF NITRO COMPOUNDS (R-NO2) OR NITROSO COMPOUNDS (R-NO) CONTAINING SULPHONIC OR CARBOXYLIC GROUP INTO CORRESPONDING AMINO COMPOUNDS (R-NH2) WITH INHERENT RECYCLE OF ALL ACIDIC STREAMS GENERATED IN SYNTHESIS<br/>[FR] PROCÉDÉ CHIMIQUE ÉCOLOGIQUE POUR LA RÉDUCTION DE COMPOSÉS NITRO (R-NO2) OU DE COMPOSÉS NITROSO (R-NO) CONTENANT UN GROUPE SULFONIQUE OU CARBOXYLIQUE EN COMPOSÉS AMINO CORRESPONDANTS (R-NH2) AVEC UN RECYCLAGE INHÉRENT DE TOUS LES COURANTS ACIDES PRODU申请人:PADIA BHADRESH K公开号:WO2011048535A1公开(公告)日:2011-04-28The process of the present invention creates a sustainable and closed water loop allowing inherent recycles of all liquid streams generated in the process. The liquid streams generated during the process of the invention are inherently recycled completely, making the process of the present invention a zero liquid discharge process which is environmentally friendly and sustainable. This invention further relates to a sustainable chemical process of reduction of R- NO2 or R-NO into corresponding R-NH2 that produces environmentally friendly R-NH2 in good yields and selectivity with large of mother liquor recycle. The process has a wide scope in that it can be applied to a number of molecules.
-
A novel application of the Diels–Alder reaction: nitronaphthalenes as normal electron demand dienophiles作者:Elisa Paredes、Romina Brasca、María Kneeteman、Pedro M.E. ManciniDOI:10.1016/j.tet.2007.02.054日期:2007.4detected as main product, suggesting that a competitive reaction would probably take place. The results clearly confirmed the dienophilic nature of nitronaphthalenic double bonds and provided an alternative procedure for phenanthrene derivatives and N-naphthylpyrroles' synthesis. The relative reactivity of the reactants and the viability of the reactions were discussed from a theoretical point of view.
-
A novel and efficient synthesis of phenanthrene derivatives via palladium/norbornadiene-catalyzed domino one-pot reaction作者:Yue Zhong、Wen-Yu Wu、Shao-Peng Yu、Tian-Yuan Fan、Hai-Tao Yu、Nian-Guang Li、Zhi-Hao Shi、Yu-Ping Tang、Jin-Ao DuanDOI:10.3762/bjoc.15.26日期:——Herein we report a novel palladium-catalyzed reaction that results in phenanthrene derivatives using aryl iodides, ortho-bromobenzoyl chlorides and norbornadiene in one pot. This dramatic transformation undergoes ortho-C–H activation, decarbonylation and subsequent a retro-Diels–Alder process. Pleasantly, this protocol has a wider substrate range, shorter reaction times and higher yields of products
表征谱图
-
氢谱1HNMR
-
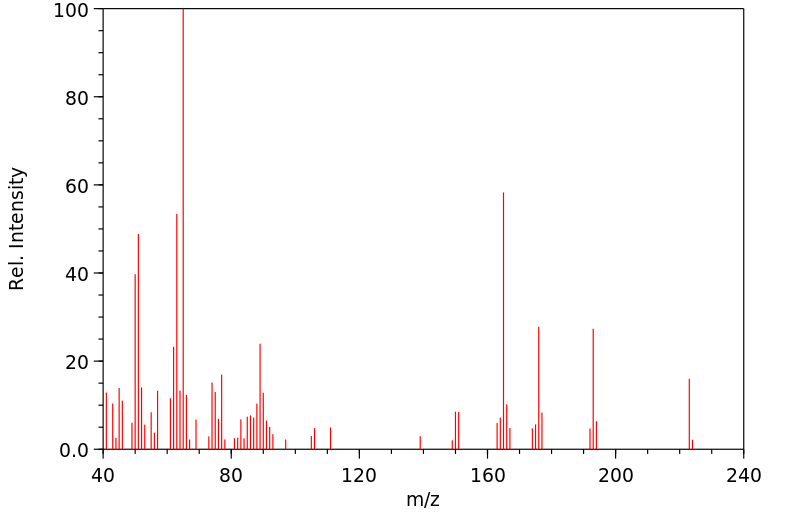
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩