1-硫代异氰酸戊酯 | 629-12-9
中文名称
1-硫代异氰酸戊酯
中文别名
1-戊基异硫氰酸酯;异硫氰酸戊酯;1-异硫代氰酸戊酯
英文名称
pentyl isothiocyanate
英文别名
n-pentyl isothiocyanate;1-isothiocyanatopentane
CAS
629-12-9
化学式
C6H11NS
mdl
MFCD00014444
分子量
129.226
InChiKey
SGHJUJBYMSVAJY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:95 °C
-
密度:0.93
-
闪点:32°C
-
LogP:3.06
-
物理描述:Colourless to yellow liquid; Sharp green irritating aroma
-
溶解度:Very slightly soluble in water; freely soluble in ether
-
折光率:1.495-1.501
-
保留指数:1077
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:8
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:44.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:8
-
危险类别码:R10
-
危险品运输编号:3080
-
包装等级:III
-
危险类别:8
-
安全说明:S26,S36/37/39
-
储存条件:应将化学品存放在充满干燥惰性气体的容器中,并置于阴凉、干燥处。储存地点需加锁,钥匙由技术人员及其助手管理,以确保安全。请避免接触湿气和水分,并远离氧化剂及酸类物质。
SDS
| Name: | Pentyl isothiocyanate 95+% Material Safety Data Sheet |
| Synonym: | n-Amylisothiocyanat |
| CAS: | 629-12-9 |
Synonym:n-Amylisothiocyanat
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 629-12-9 | Pentyl isothiocyanate | 95+% | 211-075-6 |
Risk Phrases: 20/21/22 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed. Causes burns.Moisture sensitive.
Potential Health Effects
Eye:
Causes eye burns. Lachrymator (substance which increases the flow of tears).
Skin:
Harmful if absorbed through the skin. Causes skin burns.
Ingestion:
Harmful if swallowed. Causes gastrointestinal tract burns.
Inhalation:
Harmful if inhaled. Causes chemical burns to the respiratory tract.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do not induce vomiting. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Corrosives area. Store under nitrogen.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 629-12-9: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 117 - 119 deg C @70mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 0.93
Molecular Formula: C6H11NS
Molecular Weight: 129
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials, exposure to moist air or water.
Incompatibilities with Other Materials:
Bases, amines, strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 629-12-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Pentyl isothiocyanate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE LIQUID, TOXIC, N.O.S.*
Hazard Class: 8 (6.1)
UN Number: 2922
Packing Group: III
IMO
Shipping Name: CORROSIVE LIQUID, TOXIC, N.O.S.
Hazard Class: 8 (6.1)
UN Number: 2922
Packing Group: III
RID/ADR
Shipping Name: CORROSIVE LIQUID, TOXIC, N.O.S.
Hazard Class: 8 (6.1)
UN Number: 2922
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 34 Causes burns.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 629-12-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 629-12-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 629-12-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:季巴比妥酸的催化不对称合成摘要:前手性巴比妥酸(假对称 1,3-二酰胺的一种亚型)的催化不对称 α-官能化产生相应的 5,5-二取代(季)衍生物基本上仍未解决。在这项研究中,2-烷硫基-4,6-二氧嘧啶被设计为关键的 1,3-二酰胺替代物,在胺-方酸酰胺催化的 CC 键形成反应中表现出色,乙烯基酮或 Morita-Baylis-Hillmann 型烯丙基溴作为亲电子试剂。加合物的温和酸水解以前所未有的对映选择性提供具有环内季碳的巴比妥酸衍生物,为生物学评估提供有价值的材料。DOI:10.1021/jacs.7b09124
-
作为产物:描述:参考文献:名称:脂肪族异硫氰酸酯类似物作为抗生素的合成及其构效关系摘要:异硫氰酸盐(ITC)是植物中发现的芥子油苷的多种分解产物之一,并且具有针对各种病原体的生物活性。在这项工作中,制备了脂族异硫氰酸酯,并测试了其对植物病原性真菌和细菌的抗菌活性,以了解其结构与活性之间的关系。结果表明施加空间抑制该长链衍生物关于对信托公司的毒性水稻纹枯病菌由于位阻和八个脂族的ITC的顺序为乙基>的 Ñ丙基>甲基> ñ -己基> Ñ辛基> ñ -丁基> 正庚基> n-戊基。由于ITC的疏水性通过增加烷基链长度而增强,因此ITC对胡萝卜欧文氏菌的抗菌活性中等,随着疏水性的增加而增强,其顺序为正辛基> 正戊基> 正庚基> 正己基> n丙基> 正丁基>甲基>乙基。目前的研究表明,一些化合物表现出令人信服的抗菌活性,可以作为控制茄螺和胡萝卜螺的传统合成杀菌剂的可接受替代品。DOI:10.1007/s00044-012-0323-4
文献信息
-
Novel pyridine derivative and pyrimidine derivative申请人:Matsushima Tomohiro公开号:US20050277652A1公开(公告)日:2005-12-15A compound represented by the following formula, a salt thereof or a hydrate of the foregoing has an excellent hepatocyte growth factor receptor (HGFR) inhibitory activity, and exhibits anti-tumor activity, angiogenesis inhibitory activity and cancer metastasis inhibitory activity. [R 1 represents C 1-6 alkyl or the like; R 2 and R 3 represent hydrogen; R 4 , R 5 , R 6 , and R 7 may be the same or different and each represents hydrogen, halogen, C 1-6 alkyl or the like; R 8 represents hydrogen or the like; R 9 represents C 1-6 alkyl or the like; V 1 represents oxygen or the like; V 2 represents oxygen or sulfur; W represents —NH— or the like; X represents —CH═, nitrogen or the like; and Y represents oxygen or the like.]
-
Pegylated non-hypertensive hemoglobins, methods of preparing same, and uses thereof申请人:Acharya A. Seetharama公开号:US20050159339A1公开(公告)日:2005-07-21The present invention provides pegylated hemoglobins comprising a thiocarbamoyl-phenyl-polyethylene glycol (PEG) attached to hemoglobin, and comprising a polyethylene glycol (PEG) attached to hemoglobin by an acyl group. The invention also provides methods of preparing pegylated hemoglobins using isothiocyanato phenyl carbamate of PEG and using isothiocyanato phenyl di-PEG carbamate. The invention further provides compositions and blood substitutes comprising pegylated hemoglobins and methods of treating a subject which comprise administering to the subject blood substitutes comprising non-hypertensive pegylated hemoglobins.
-
Novel ligands for the hisb10 zn2+ sites of the r-state insulin hexamer
-
Heteroaryl compounds as P2Y1 receptor inhibitors申请人:Sutton C. James公开号:US20060173002A1公开(公告)日:2006-08-03The present invention provides novel heteroaryl compounds and analogues thereof, which are selective inhibitors of the human P2Y 1 receptor. The invention also provides for various pharmaceutical compositions of the same and methods for treating diseases responsive to modulation of P2Y 1 receptor activity.本发明提供了新颖的杂环芳基化合物及其类似物,这些化合物是人类P2Y1受体的选择性抑制剂。该发明还提供了相应的各种药物组合物以及调节P2Y1受体活性治疗对其敏感的疾病的方法。
-
Aminobenzoxazoles as therapeutic agents申请人:Wishart Neil公开号:US20060025383A1公开(公告)日:2006-02-02A compound of Formula (I), wherein the substituents are as defined herein, which are useful as kinase inhibitors.其中取代基如本文所述的公式(I)的化合物,用作激酶抑制剂。
表征谱图
-
氢谱1HNMR
-
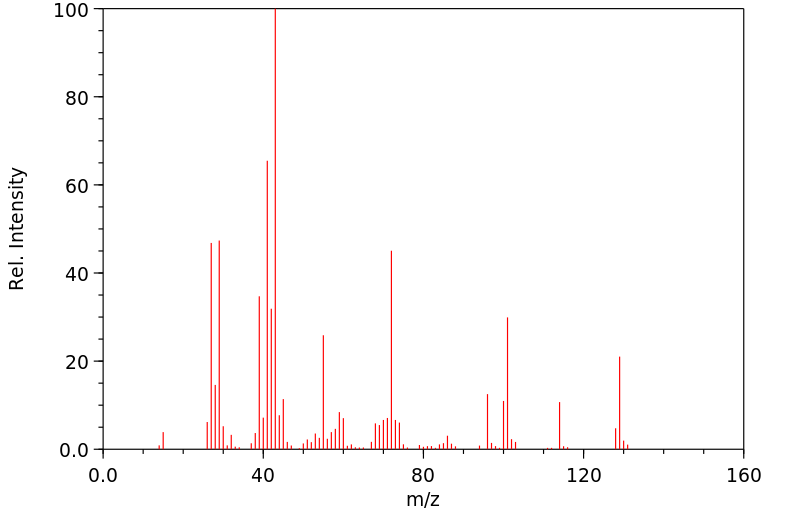
质谱MS
-
碳谱13CNMR
-
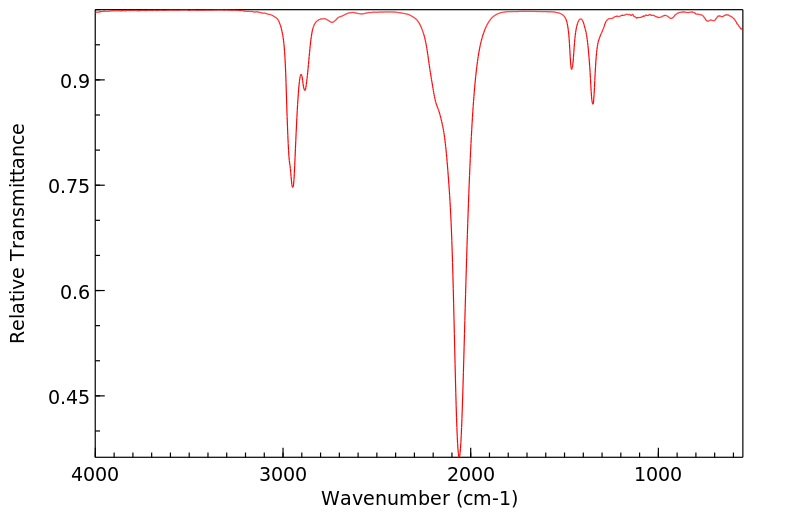
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-异硫氰基-1-环戊羧酸乙酯
顺-2-异硫氰基-1-环己烷羧酸乙酯
羰基异氰酸酯异硫氰酸酯
羰基二异硫氰酸酯
硫代异氰酸环戊酯
甲代烯丙基异硫氰酸酯
环己烷羰基异硫氰酸酯
环己基异硫氰酸脂
环丙基甲基异硫氰酸酯
环丁烷羰基异硫氰酸酯
异硫氰酸甲酯
异硫氰酸甲氧基甲酯
异硫氰酸甲基环己酯
异硫氰酸环辛酯
异硫氰酸环庚脂
异硫氰酸环十二酯
异硫氰酸环丙酯
异硫氰酸烯丙酯
异硫氰酸溴代乙酯
异硫氰酸氯代乙酯
异硫氰酸氨基甲酰
异硫氰酸异戊酯
异硫氰酸异丙酯
异硫氰酸异丁酯
异硫氰酸己酯
异硫氰酸叔辛酯
异硫氰酸十六酯
异硫氰酸十一烷酯
异硫氰酸仲丁酯
异硫氰酸乙酰酯
异硫氰酸乙酯
异硫氰酸乙烯
异硫氰酸3-丁烯酯
异硫氰酸2-甲氧基乙酯
异硫氰酸1-金刚烷酯
异硫氰酰甲酸甲酯
异硫氰酰甲酸乙酯
异硫氰基环丁烷
异硫代氰酰基乙醛二甲基乙缩醛
异氰酸丙酯
己烷,1-[(2-异硫氰基乙基)硫代]-
己烯,1-异硫氰基-
天然芥菜籽油
叔戊基异硫氰酸酯
叔丁基异硫氰酸酯
双(1-异硫氰基乙基)醚
十八烷基异氰酸酯
二(异硫氰酰甲基)醚
丙脒,N-丁基-2-氯-
三芥子酸甘油酯