异硫氰酸乙酯 | 542-85-8
中文名称
异硫氰酸乙酯
中文别名
乙基芥子油;乙基乙酸丙烯酯;乙芥子油;乙基异硫氰酸酯;异氰酸乙酯
英文名称
Ethyl isothiocyanate
英文别名
isothiocyanatoethane
CAS
542-85-8
化学式
C3H5NS
mdl
MFCD00004820
分子量
87.1454
InChiKey
HBNYJWAFDZLWRS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-6 °C
-
沸点:130-132 °C(lit.)
-
密度:0.995 g/mL at 25 °C(lit.)
-
闪点:90 °F
-
溶解度:氯仿(少量溶解)、乙酸乙酯(可溶)
-
介电常数:19.7(20℃)
-
LogP:1.47 at 25℃ and pH7
-
物理描述:Colourless liquid; Sharp mustard-like aroma
-
蒸汽压力:11.42 mmHg
-
折光率:1.510-1.515
-
保留指数:786.2;805.9
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:5
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:44.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S23,S26,S36/37/39,S45,S61
-
危险类别码:R23/24/25,R10,R34
-
WGK Germany:3
-
海关编码:2930909090
-
危险品运输编号:UN 3286 3/PG 2
-
危险类别:6.1
-
RTECS号:NX8590000
-
包装等级:II
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,库温不宜超过30℃。 - 保持容器密封。 - 应与氧化剂、酸类、碱类、食用化学品分开存放,切忌混储。 - 采用防爆型照明和通风设施。 - 禁止使用易产生火花的机械设备和工具。 - 储区应备有泄漏应急处理设备和合适的收容材料。
制备方法与用途
用途:用于制药和制作杀虫剂。
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Freund; Asbrand, Justus Liebigs Annalen der Chemie, 1895, vol. 285, p. 192摘要:DOI:
-
作为产物:描述:N-乙基二硫代氨基甲酸 在 三光气 作用下, 以 Petroleum ether 为溶剂, 反应 2.0h, 以92%的产率得到异硫氰酸乙酯参考文献:名称:1-乙基-(3-二甲基氨基丙基)碳二亚胺盐酸盐的制备方法摘要:本发明涉及一种1‑乙基‑(3‑二甲基氨基丙基)碳二亚胺盐酸盐的制备方法,属于医药中间体技术领域。所述的制备方法包含反应生成EDTC、制得EITC粗品、蒸馏分馏、制得中间体、制得EDC和制得目标产物等七个步骤。本发明所提供的制备方法,具有高的产品收率和纯度,操作步骤简单,易于实施,适合工业化生产的特点。公开号:CN109369458A
-
作为试剂:参考文献:名称:Povarov, L.S.; Vlasenkova, L.I.; Lazhko, E.I., Russian Journal of Bioorganic Chemistry, 1995, vol. 21, # 8, p. 551 - 554摘要:DOI:
文献信息
-
[EN] THIOPHENE DERIVATIVES FOR THE TREATMENT OF DISORDERS CAUSED BY IGE<br/>[FR] DÉRIVÉS DE THIOPHÈNE POUR LE TRAITEMENT DE TROUBLES PROVOQUÉS PAR IGE申请人:UCB BIOPHARMA SRL公开号:WO2019243550A1公开(公告)日:2019-12-26Thiophene derivatives of formula (I) and a pharmaceutically acceptable salt thereof are provided. These compounds have utility for the treatment or prevention of disorders caused by IgE, such as allergy, type 1 hypersensitivity or familiar sinus inflammation.
-
Studying Products of Hydrazine Interaction with Isothiocyanates by Means of Chromatography and Mass Spectrometry作者:A. V. Ul’yanov、K. E. Polunin、I. A. Polunina、A. K. BuryakDOI:10.1134/s0036024421050290日期:2021.5compounds in real-time and delayed modes are optimized. The physicochemical characteristics of the sorption of thiosemicarbazides are determined. The decomposition and fragmentation of their metastable protonated molecules are studied. Schemes are proposed for the formation of fragmented and characteristic thiosemicarbazide ions in different modes of ionization.
-
Combination of FBPase inhibitors and insulin sensitizers for the treatment of diabetes申请人:Metabasis Therapeutics, Inc.公开号:US06756360B1公开(公告)日:2004-06-29Pharmaceutical compositions containing an FBPase inhibitor and an insulin sensitizer are provided as well as methods for treating diabetes and diseases responding to increased glycemic control, an improvement in insulin sensitivity, a reduction in insulin levels, or an enhancement of insulin secretion.
-
Diphenyl ether derivatives and herbicides containing same申请人:Mitsui Toatsu Chemicals, Inc.公开号:US04515628A1公开(公告)日:1985-05-07Novel diphenyl ether hydrazine derivatives, particularly 2-nitro-5 (nucleus-substituted phenoxy) phenylhydrazine derivatives are provided. They are useful as a selective herbicide having a high herbicidal acitivity and residual efficacy.
-
Design, synthesis, and structure–activity relationship of carbamate-tethered aryl propanoic acids as novel PPARα/γ dual agonists作者:Nam-Jung Kim、Kwang-Ok Lee、Bon-Woong Koo、Funan Li、Ja-Kyung Yoo、Hyun-Ju Park、Kyung-Hoon Min、Joong In Lim、Mi Kyung Kim、Jin-Kwan Kim、Young-Ger SuhDOI:10.1016/j.bmcl.2007.04.057日期:2007.7dual agonists, which show excellent agonistic activity in PPARalpha/gamma transactivation assay. In particular, (R)-9d was identified as a potent PPARalpha/gamma dual agonist with EC(50)s of 0.377 microM in PPARalpha and 0.136 microM in PPARgamma, respectively. Interestingly, the structure-activity relationship revealed that the stereochemistry of the identified PPARalpha/gamma dual agonists significantly
表征谱图
-
氢谱1HNMR
-
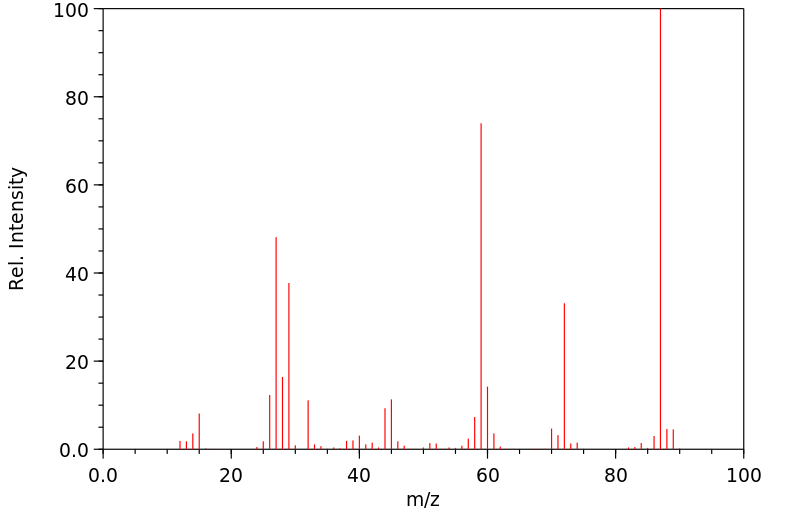
质谱MS
-
碳谱13CNMR
-
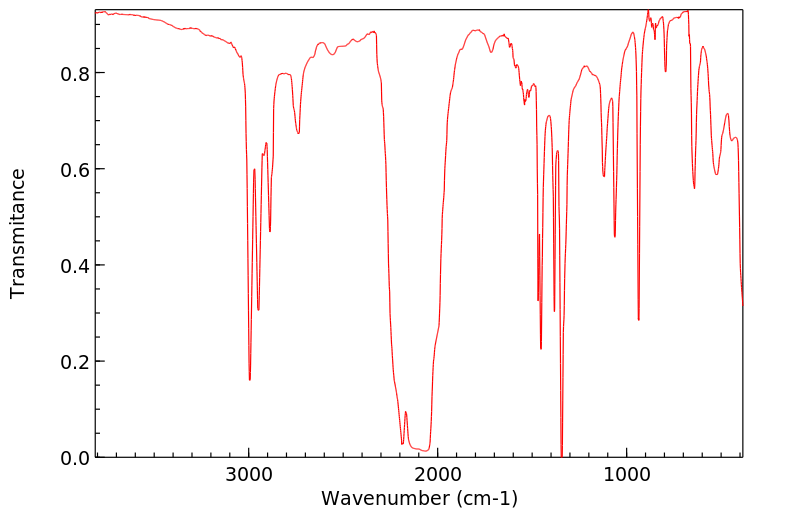
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-异硫氰基-1-环戊羧酸乙酯
顺-2-异硫氰基-1-环己烷羧酸乙酯
羰基异氰酸酯异硫氰酸酯
羰基二异硫氰酸酯
硫代异氰酸环戊酯
甲代烯丙基异硫氰酸酯
环己烷羰基异硫氰酸酯
环己基异硫氰酸脂
环丙基甲基异硫氰酸酯
环丁烷羰基异硫氰酸酯
异硫氰酸甲酯
异硫氰酸甲氧基甲酯
异硫氰酸甲基环己酯
异硫氰酸环辛酯
异硫氰酸环庚脂
异硫氰酸环十二酯
异硫氰酸环丙酯
异硫氰酸烯丙酯
异硫氰酸溴代乙酯
异硫氰酸氯代乙酯
异硫氰酸氨基甲酰
异硫氰酸异戊酯
异硫氰酸异丙酯
异硫氰酸异丁酯
异硫氰酸己酯
异硫氰酸叔辛酯
异硫氰酸十六酯
异硫氰酸十一烷酯
异硫氰酸仲丁酯
异硫氰酸乙酰酯
异硫氰酸乙酯
异硫氰酸乙烯
异硫氰酸3-丁烯酯
异硫氰酸2-甲氧基乙酯
异硫氰酸1-金刚烷酯
异硫氰酰甲酸甲酯
异硫氰酰甲酸乙酯
异硫氰基环丁烷
异硫代氰酰基乙醛二甲基乙缩醛
异氰酸丙酯
己烷,1-[(2-异硫氰基乙基)硫代]-
己烯,1-异硫氰基-
天然芥菜籽油
叔戊基异硫氰酸酯
叔丁基异硫氰酸酯
双(1-异硫氰基乙基)醚
十八烷基异氰酸酯
二(异硫氰酰甲基)醚
丙脒,N-丁基-2-氯-
三芥子酸甘油酯