硫代异氰酸环戊酯 | 33522-03-1
中文名称
硫代异氰酸环戊酯
中文别名
异硫氰酸环戊酯;环戊基异硫氰酸酯
英文名称
cyclopentyl isothiocyanate
英文别名
isothiocyanatocyclopentane;Cyclopentylisothiocyanat
CAS
33522-03-1
化学式
C6H9NS
mdl
MFCD00040877
分子量
127.21
InChiKey
PJOODZCPFADLCI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:94 °C
-
密度:1.03
-
闪点:75-76°C/10mm
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知危险反应。应避免与氧化物、水分等接触。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:8
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.833
-
拓扑面积:44.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:8
-
危险品标志:Xn
-
危险类别码:R20/21/22,R36/37/38
-
危险品运输编号:2810
-
海关编码:2930909090
-
包装等级:III
-
危险类别:8
-
安全说明:S26,S36/37/39
-
WGK Germany:3
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。确保工作环境有良好的通风或排气设施。
SDS
| Name: | Cyclopentyl isothiocyanate 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 33522-03-1 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 33522-03-1 | Cyclopentyl isothiocyanate | 97% | unlisted |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.Moisture sensitive.
Potential Health Effects
Eye:
Causes eye irritation. Lachrymator (substance which increases the flow of tears).
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Store under nitrogen.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 33522-03-1: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 92 - 94 deg C @15mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.03
Molecular Formula: C6H9NS
Molecular Weight: 127
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials, exposure to moist air or water.
Incompatibilities with Other Materials:
Bases, oxidizing agents, reducing agents, amines.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, oxides of sulfur, carbon dioxide, acrid smoke and fumes.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 33522-03-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Cyclopentyl isothiocyanate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
IMO
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
RID/ADR
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 33522-03-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 33522-03-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 33522-03-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:Onisi, Yakugaku Zasshi/Journal of the Pharmaceutical Society of Japan, 1959, vol. 79, p. 559,564摘要:DOI:
-
作为产物:参考文献:名称:Nature of the carbonium ion. VIII. Cycloalkyl cations from thiocyanate isomerizations摘要:DOI:10.1021/jo00973a019
文献信息
-
[EN] TRIAZOLE FURAN COMPOUNDS AS AGONISTS OF THE APJ RECEPTOR<br/>[FR] COMPOSÉS DE TRIAZOLE FURANE UTILISÉS EN TANT QU'AGONISTES DU RÉCEPTEUR APJ申请人:AMGEN INC公开号:WO2018097944A1公开(公告)日:2018-05-31Compounds of Formula (I) and Formula (II), pharmaceutically acceptable salt thereof, stereoisomers of any of the foregoing, or mixtures thereof are agonists of the APJ Receptor and have use in treating cardiovascular and other conditions. Compounds of Formula (I) and Formula (II) have the following structures: (I); (II). Intermediates (V) are also claimed.式(I)和式(II)的化合物,其药用盐,任何上述化合物的立体异构体,或它们的混合物是APJ受体的激动剂,并且在治疗心血管和其他疾病方面有用。式(I)和式(II)的化合物具有以下结构:(I); (II)。中间体(V)也被要求。
-
(Propargylsulfanyl)-2-aza-1,3,5-trienes as a direct source for novel family of highly functionalized 4,5-dihydro-1,3-thiazoles作者:Nina A. Nedolya、Ol'ga A. Tarasova、Alexander I. Albanov、Lyudmila V. Klyba、Boris A. TrofimovDOI:10.1016/j.tet.2016.12.064日期:2017.22-(1-alkoxyprop-1-enyl)-5-ethenylidene-4,5-dihydro-1,3-thiazoles has been disclosed through the reaction of (propargylsulfanyl)-2-aza-1,3,5-trienes (readily available from alkoxyallenes, isothiocyanates, and propargyl bromide) with potassium or sodium tert-butoxide (1.1–1.4 equiv) under mild conditions [DMSO/THF (∼1:4–5), ca. −30 °C, 15–30 min]. The process proceeds through deprotonation of an activated SCH2 group
-
Mass spectra of new heterocycles: X. Fragmentation of the molecular ions of 1-alkyl(cycloalkyl, aryl)-3-alkoxy(aryl)-2-methylsulfanyl-1H-pyrroles作者:L. V. Klyba、N. A. Nedolya、O. A. Tarasova、E. R. Zhanchipova、O. G. VolostnykhDOI:10.1134/s1070428010070134日期:2010.7The mass spectra of previously unknown 1-alkyl(cycloalkyl, aryl)-3-alkoxy(aryl)-2-methylsulfanyl-1H-pyrroles were studied. Fragmentation of all 3-alkoxy-substituted pyrroles under electron impact (70 eV) follow both ether and sulfide decomposition paths; In particular, 1-R-substituted 3-methoxy-2-methylsulfanyl-1H-pyrroles (R = Me, Et, i-Pr, s-Bu, cyclo-C5H9, cyclo-C6H11, Ph) lose methyl radical group研究了以前未知的1-烷基(环烷基,芳基)-3-烷氧基(芳基)-2-甲基硫基-1 H-吡咯的质谱。所有3-烷氧基取代的吡咯在电子轰击(70 eV)下的裂解均遵循醚和硫化物的分解路径。特别是1-R-取代的3-甲氧基-2-甲基硫烷基-1 H-吡咯(R = Me,Et,i- Pr,s- Bu,环-C 5 H 9,环-C 6 H 11,Ph )从甲氧基和甲基硫烷基基团失去甲基基团。1秒的质谱丁基和1环烷基吡咯也有一个强峰(10-49%),该峰来自通过同步氢转移裂解NR键形成的奇电子[ M C n H 2 n ] +·离子。3-烷氧基-1-异丙基-2-甲基硫烷基-1 H-吡咯(Alk = Et,i -Pr,t -Bu)断裂中的O-Alk键断裂伴随重排过程,导致相应的烯烃和1-电子的1-异丙基-2-甲基硫烷基-1 H-吡咯-3-醇离子。1-烷基-2-甲基硫烷基-3-苯基-1 H-吡咯的主要裂解途径(Alk
-
C ring may be dispensable for β-carboline: Design, synthesis, and bioactivities evaluation of tryptophan analog derivatives based on the biosynthesis of β-carboline alkaloids作者:Yuanqiong Huang、Yongxian Liu、Yuxiu Liu、Hongjian Song、Qingmin WangDOI:10.1016/j.bmc.2015.08.016日期:2016.2According to our previous work and the latest research on the biosynthesis of β-carboline, and using the reverse thinking strategy, tryptophan, the biosynthesis precursor of β-carboline alkaloids, and their derivatives were synthesized, and their biological activities and structure–activity relationships were studied. This bioassay showed that these compounds exhibited good inhibitory activities against根据我们先前对β-咔啉生物合成的研究和最新研究,并采用逆向思维策略,合成了色氨酸,β-咔啉生物碱的生物合成前体及其衍生物,以及它们的生物活性和构效关系被研究了。这项生物测定结果表明,这些化合物对烟草花叶病毒(TMV)表现出良好的抑制活性。尤其是(S)-2-氨基-3-(1 H-吲哚-3-基)-N-辛基丙酰胺(4)(63.3±2.1%,67.1±1.9%,68.7±1.3%和64.5±3.1%, 500μg/ mL)在体外和体内均表现出最佳的抗病毒活性。化合物4选择该化合物用于野外试验和急性口服毒性试验,结果表明该化合物在野外具有良好的抗TMV活性,而急性口服毒性较低。我们还发现这些化合物显示出抗真菌活性和杀虫活性。
-
A One-Pot Assembly of Fully Substituted Alkyl 5-Aminothiophene-2-carboxylates from Allenes, Isothiocyanates, and Alkyl 2-Bromoacetates作者:Nina A. Nedolya、Ol’ga A. Tarasova、Alexander I. Albanov、Boris A. TrofimovDOI:10.1021/acs.joc.7b01217日期:2017.7.21A novel simple approach to highly functionalized multisubstituted thiophenes such as alkyl 4-alkoxy-5-amino-3-methylthiophene-2-carboxylates through the one-pot sequential reaction of α-lithiated alkoxyallenes with isothiocyanates and alkyl 2-bromoacetates has been discovered. The process proceeds quickly (30–45 min) via in situ formation and intramolecular cyclization of alkyl 2-[(2-alkoxybuta-2,
表征谱图
-
氢谱1HNMR
-
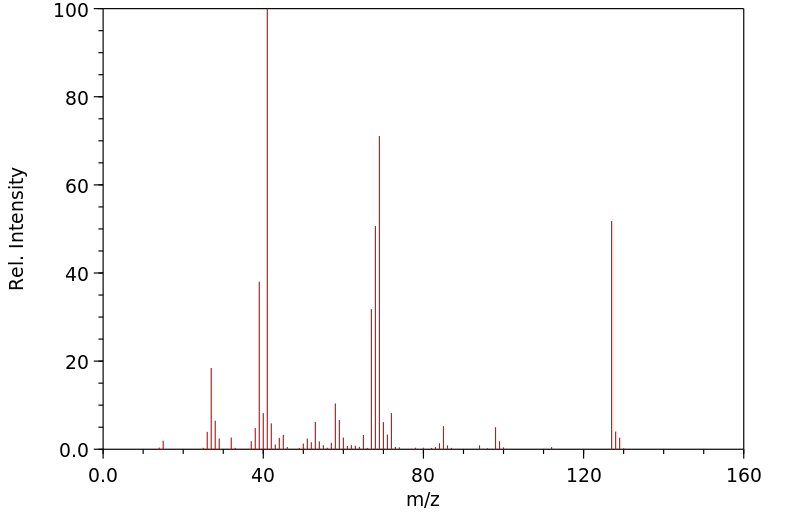
质谱MS
-
碳谱13CNMR
-
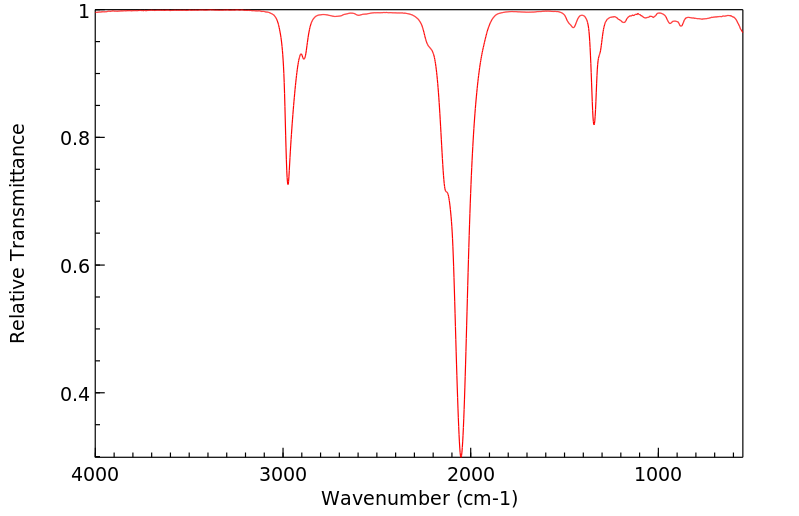
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-异硫氰基-1-环戊羧酸乙酯
顺-2-异硫氰基-1-环己烷羧酸乙酯
羰基异氰酸酯异硫氰酸酯
羰基二异硫氰酸酯
硫代异氰酸环戊酯
甲代烯丙基异硫氰酸酯
环己烷羰基异硫氰酸酯
环己基异硫氰酸脂
环丙基甲基异硫氰酸酯
环丁烷羰基异硫氰酸酯
异硫氰酸甲酯
异硫氰酸甲氧基甲酯
异硫氰酸甲基环己酯
异硫氰酸环辛酯
异硫氰酸环庚脂
异硫氰酸环十二酯
异硫氰酸环丙酯
异硫氰酸烯丙酯
异硫氰酸溴代乙酯
异硫氰酸氯代乙酯
异硫氰酸氨基甲酰
异硫氰酸异戊酯
异硫氰酸异丙酯
异硫氰酸异丁酯
异硫氰酸己酯
异硫氰酸叔辛酯
异硫氰酸十六酯
异硫氰酸十一烷酯
异硫氰酸仲丁酯
异硫氰酸乙酰酯
异硫氰酸乙酯
异硫氰酸乙烯
异硫氰酸3-丁烯酯
异硫氰酸2-甲氧基乙酯
异硫氰酸1-金刚烷酯
异硫氰酰甲酸甲酯
异硫氰酰甲酸乙酯
异硫氰基环丁烷
异硫代氰酰基乙醛二甲基乙缩醛
异氰酸丙酯
己烷,1-[(2-异硫氰基乙基)硫代]-
己烯,1-异硫氰基-
天然芥菜籽油
叔戊基异硫氰酸酯
叔丁基异硫氰酸酯
双(1-异硫氰基乙基)醚
十八烷基异氰酸酯
二(异硫氰酰甲基)醚
丙脒,N-丁基-2-氯-
三芥子酸甘油酯