1-苯基吡唑并[3,4-d]嘧啶 | 53645-79-7
中文名称
1-苯基吡唑并[3,4-d]嘧啶
中文别名
——
英文名称
1-phenyl-1H-pyrazolo[3,4-d]pyrimidine
英文别名
1-phenylpyrazolo[3,4-d]pyrimidine;1-phenyl-1H-pyrazolo[3,4-d]pyrimidine
CAS
53645-79-7
化学式
C11H8N4
mdl
——
分子量
196.211
InChiKey
BDIXKUKUFWHLIO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:15
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:43.6
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氯-1-苯基-1H-吡唑并[3,4-d]嘧啶 4-chloro-1-(phenyl)-1H-pyrazolo[3,4-d]pyrimidine 5334-48-5 C11H7ClN4 230.656 1-苯基吡唑并[3,4-d]嘧啶-4-腈 1-phenyl-1H-pyrazolo[3,4-d]pyrimidine-4-carbonitrile 65143-08-0 C12H7N5 221.221 —— 1-Phenyl-1H-pyrazolo<3,4-d>pyrimidin-4-carbonsaeure 68380-49-4 C12H8N4O2 240.221 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-羟基-1-苯基吡唑并[3,4-D]嘧啶 1-phenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one 21314-17-0 C11H8N4O 212.211 1-苯基吡唑并[3,4-d]嘧啶-4-腈 1-phenyl-1H-pyrazolo[3,4-d]pyrimidine-4-carbonitrile 65143-08-0 C12H7N5 221.221 —— phenyl(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yl)methanol 59563-79-0 C18H14N4O 302.335 —— phenyl-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yl)-methanone 59563-84-7 C18H12N4O 300.319
反应信息
-
作为反应物:描述:参考文献:名称:Higashino, Takeo; Sato, Susumu; Miyashita, Akira, Chemical and pharmaceutical bulletin, 1986, vol. 34, # 11, p. 4569 - 4576摘要:DOI:
-
作为产物:描述:参考文献:名称:Dehalogenation and Barbier-Type Hydroxyalkylation of π-Deficient Haloheterocycles Using Indium摘要:The reaction of pi-deficient haloheterocycles with indium metal in water gave corresponding dehalogenated heterocycles. The use of diluted hydrochloric acid instead of water accelerated the reductive reactivity of indium metal. Furthermore, Barbier-type additions proceeded by reactions of alpha-iodoheterocycles with indium in the presence of pivalaldehyde.DOI:10.3987/com-08-s(f)95
文献信息
-
Carbon-Carbon Bond Cleavage of .ALPHA.-Hydroxybenzylheteroarenes Catalyzed by Cyanide Ion: Retro-Benzoin Condensation Affords Ketones and Heteroarenes and Benzyl Migration Affords Benzylheteroarenes and Arenecarbaldehydes.作者:Yumiko SUZUKI、Yuki TAKEMURA、Ken-ichi IWAMOTO、Takeo HIGASHINO、AKira MIYASHITADOI:10.1248/cpb.46.199日期:——4-(α-Benzyl-α-hydroxybenzyl)quinazoline (4a) underwent retro-benzoin condensation catalyzed by cyanide ion to give deoxybenzoin (2a) and quinazoline (5a). Similarly, several nitrogen-containing heteroarenes (4, 9, 12, 16-19) having an α-hydroxybenzyl group at the α-position of the nitrogen underwent retro-benzoin type condensation to afford ketones (2) and heteroarenes (5). However, similar reaction of pyrazolopyrimidines (13, 14, 15) having an α-benzyl-α-hydroxybenzyl group resulted in benzyl migration, giving benzylpyrazolopyrimidines (8) and arenecarbaldehydes (3). Tetrabutylammonium cyanide (11, Bu4NCN) was a more effective cyanide ion donor than KCN (10). The retro-benzoin condensation was applied to the synthesis of 2-substituted quinazolines (38) from 2-chloro-4-aroylquinazolines (34), using the aroyl group as a protecting and electron-withdrawing group.4-(α-苄基-α-羟基苄基)喹唑啉(4a)在氰离子催化下经历逆苯偶姻缩合反应,生成脱氧苯偶姻(2a)和喹唑啉(5a)。同样,多个含氮杂环芳烃(4, 9, 12, 16-19)在其氮原子的α位上具有α-羟基苄基的结构,也经历逆苯偶姻型缩合反应,生成了酮(2)和杂环芳烃(5)。然而,具有α-苄基-α-羟基苄基结构的吡唑嘧啶(13, 14, 15)在类似的反应中引发了苄基迁移,生成了苄基吡唑嘧啶(8)和芳烃羰醛(3)。四丁基铵氰(11, Bu4NCN)比KCN(10)更有效地作为氰离子的供体。逆苯偶姻缩合反应被应用于从2-氯-4-芳酰基喹唑啉(34)合成2-取代喹唑啉(38),使用芳酰基作为保护和电子 withdrawing 基团。
-
PYRAZOLOPYRIMIDINE ANALOGS AND THEIR USE AS MTOR KINASE AND PI3 KINASE INHIBITORS申请人:Zask Arie公开号:US20080234262A1公开(公告)日:2008-09-25The present invention relates to Pyrazolopyrimidine Analogs, methods of making Pyrazolopyrimidine Analogs, compositions comprising a Pyrazolopyrimidine Analog, and methods for treating mTOR-related disorders comprising administering to a subject in need thereof an effective amount of a Pyrazolopyrimidine Analog. The invention also relates to treating PI3K-related disorders comprising administering to a subject in need thereof an effective amount of a Pyrazolopyrimidine Analog.
-
NOVEL PYRAZOLO [3, 4 -D] PYRIMIDINE DERIVATIVES AS ANTI-CANCER AGENTS申请人:Konakanchi Durga Prasad公开号:US20100298351A1公开(公告)日:2010-11-25The invention relates to substituted pyrazolo[3,4-d]pyrimidine derivatives of the Formula-(I), or pharmaceutically-acceptable salts thereof, which possess anti-proliferative activity such as anti-cancer activity and are accordingly useful in methods of treatment of the human or animal body. The invention also relates to processes for the manufacture of substituted pyrazolo[3,4-d]pyrimidine derivatives, to pharmaceutical compositions containing the compound and to its use in the manufacture of medicaments for the production of an anti-proliferative effect in a warm-blooded animal such as man.
-
Studies on the reaction of .PI.-deficient heterocycles with aromatic aldehyde in the presence of cyanide ion.作者:TAKEO HIGASHINO、MASAMI GOI、EISAKU HAYASHIDOI:10.1248/cpb.24.238日期:——Dimerization of π-deficient heterocyles by the catalytic action of cyanide ion in DMSO was carried out. Thus, reaction of quinoxaline (V), 1-phenyl-1H-pyrazolo [3, 4-d] pyrimidine (VI), 1-methyl-1H-pyrazolo [3, 4-d] pyrimidine (VII), and pyrido [2, 3-b] pyrazine (VIII) with cyanide ion gave 2, 2'-biquinoxaline (IX), 4.4'-bis [1-phenyl-1H-pyrazolo [3, 4-d] pyrimidine] (X), 4, 4'-bis [1-methyl-1H-pyrazolo [3, 4-d] pyrimidine] (XI), and 2, 2'-bipyrido [2, 3-b] pyrazine (XII), respectively, although the yields of these dimers were very poor. The formation of dimer is assumed to be oxidation following a benzoin condensation-like reaction by the catalysis of cyanide ion. Also we carried out the reaction of π-deficient heterocycles with aromatic aldehyde (XVI) in the presence of cyanide ion in DMSO, and found that the expected cross benzoin condensation-like reaction has developed. Thus, 4-isoquinolinecarbonitrile (II) reacted with XVI to give α-aryl-4-cyano-1-isoquinolinemethanol (XIX) and aryl 4-cyano-1-isoquinolyl ketone (XX) together with 1, 1'-biisoquinoline-4, 4'-dicarbonitrile (IV). Similarly, V and XVI gave α-aryl-2-quinoxalinemethanol (XXI) and aryl 2-quinoxalinyl ketone (XXII), VI and XVI afforded α-aryl-1-phenyl-1H-pyrazolo [3, 4-d] pyrimidine-4-methanol (XXX) and aryl 1-phenyl-1H-pyrazolo [3, 4-d] pyrimidin-4-yl ketone (XXXI), VII and XVI produced α-aryl-1-methyl-1H-pyrazolo [3, 4-d] pyrimidine-4-methanol (XXXIV) and aryl 1-methyl-1H-pyrazolo [3, 4-d] pyrimidin-4-yl ketone (XXXV), and VIII and XVI formed aryl 2-pyrido [2, 3-b] pyrazinyl ketone (XXXIV). It seems that XIX, XXI, XXX, and XXXIV are a formal product of a cross benzoin condensation-like reaction, and XX, XXII, XXXI, XXXV, and XXXVI are oxidation product of a formal product of the reaction. And limitation of this reaction was also discussed.在DMSO中,通过氰离子的催化作用进行了π缺乏杂环的二聚反应。因此,喹喔啉(V)、1-苯基-1H-吡唑[3, 4-d]嘧啶(VI)、1-甲基-1H-吡唑[3, 4-d]嘧啶(VII)和吡啶[2, 3-b]嘧啶(VIII)与氰离子的反应分别生成了2, 2'-双喹喔啉(IX)、4, 4'-双[1-苯基-1H-吡唑[3, 4-d]嘧啶](X)、4, 4'-双[1-甲基-1H-吡唑[3, 4-d]嘧啶](XI)和2, 2'-双吡啶[2, 3-b]嘧啶(XII),尽管这些二聚体的产率非常低。假定二聚体的形成是氰离子催化下通过类似苯醇缩合反应的氧化反应。此外,我们还在氰离子的存在下,使用DMSO进行了π缺乏杂环与香豆醛(XVI)的反应,并发现预期的交叉苯醇缩合似反应得以进行。因此,4-异喹啉腈(II)与XVI反应生成α-芳基-4-氰-1-异喹啉甲醇(XIX)和芳基4-氰-1-异喹啉酮(XX),以及1, 1'-双异喹啉-4, 4'-二腈(IV)。类似地,V与XVI反应生成α-芳基-2-喹喔啉甲醇(XXI)和芳基2-喹喔啉酮(XXII),VI与XVI生成α-芳基-1-苯基-1H-吡唑[3, 4-d]嘧啶-4-甲醇(XXX)和芳基1-苯基-1H-吡唑[3, 4-d]嘧啶-4-酮(XXXI),VII与XVI生成α-芳基-1-甲基-1H-吡唑[3, 4-d]嘧啶-4-甲醇(XXXIV)和芳基1-甲基-1H-吡唑[3, 4-d]嘧啶-4-酮(XXXV),而VIII与XVI生成芳基2-吡啶[2, 3-b]嘧啶酮(XXXVI)。看起来XIX、XXI、XXX和XXXIV是交叉苯醇缩合类似反应的形式产物,而XX、XXII、XXXI、XXXV和XXXVI是该反应形式产物的氧化产物。同时还讨论了该反应的局限性。
-
Higashino, Takeo; Sato, Susumu; Miyashita, Akira, Chemical and pharmaceutical bulletin, 1987, vol. 35, # 12, p. 4803 - 4812作者:Higashino, Takeo、Sato, Susumu、Miyashita, Akira、Katori, TatsuhikoDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
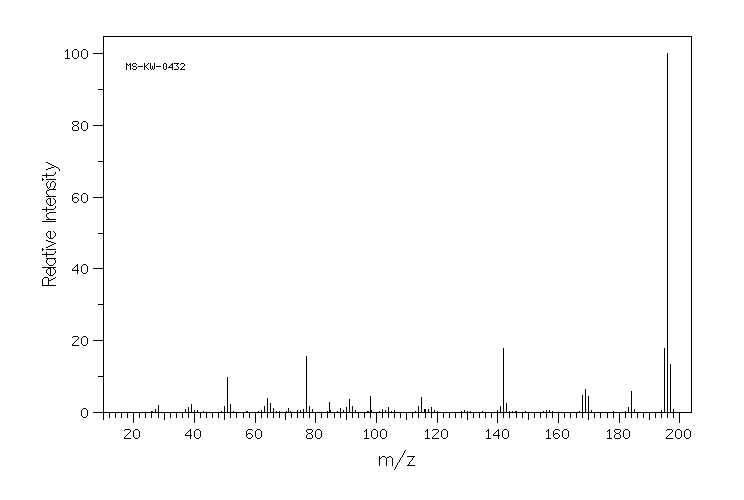
质谱MS
-
碳谱13CNMR
-
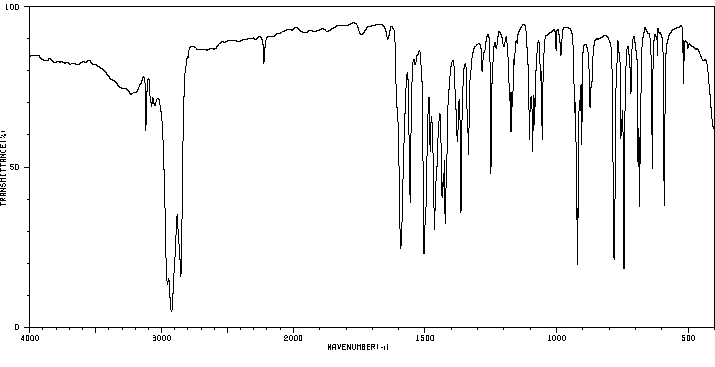
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)