10-氯-9-蒽甲醛 | 10527-16-9
中文名称
10-氯-9-蒽甲醛
中文别名
10-氯蒽-9-甲醛;1-氯-9-蒽甲醛
英文名称
10-chloro-9-anthraldehyde
英文别名
10-chloro-9-anthracenecarboxaldehyde;10-Chlor-9-anthraldehyd;10-chloro-9-anthracenecarbaldehyde;10-chloroanthracene-9-carbaldehyde
CAS
10527-16-9
化学式
C15H9ClO
mdl
MFCD00001255
分子量
240.689
InChiKey
SHYBXXMECBHHFH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:215-218 °C (lit.)
-
沸点:435.9±18.0 °C(Predicted)
-
密度:1.327±0.06 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规定使用和储存,则不会分解,未有已知危险发生。
应避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):4.5
-
重原子数:17
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
WGK Germany:3
-
海关编码:2913000090
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312,P302+P352,P304+P340,P305+P351+P338,P330,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将药品存放在密闭、阴凉干燥的地方,并确保通风良好。
SDS
| Name: | 10-Chloro-9-Anthraldehyde 97% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 10527-16-9 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 10527-16-9 | 10-Chloro-9-Anthraldehyde | 97 | 234-086-8 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 10527-16-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: yellow
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 215.00 - 218.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C15H9ClO
Molecular Weight: 240.69
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong bases, strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 10527-16-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
10-Chloro-9-Anthraldehyde - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 10527-16-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 10527-16-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 10527-16-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 10-氯蒽-9-甲醇 10-chloro-9-anthracenemethanol 19996-02-2 C15H11ClO 242.705 9-蒽甲醛 9-Anthraldehyde 642-31-9 C15H10O 206.244 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 10-氯蒽-9-甲醇 10-chloro-9-anthracenemethanol 19996-02-2 C15H11ClO 242.705 9-氯-10-氯甲基蒽 10-chloro-9-anthracenemethyl chloride 19996-03-3 C15H10Cl2 261.15 —— 9-chloro-10-vinylanthracene 38917-87-2 C16H11Cl 238.716 10-氯-9-氰基蒽 9-chloro-10-cyano-anthracene 1213-82-7 C15H8ClN 237.688 —— [(10-Chloroanthracen-9-yl)methylideneamino]urea 387846-30-2 C16H12ClN3O 297.744
反应信息
-
作为反应物:描述:参考文献:名称:合成,细胞毒性评价的人类细胞系和异Arylidene-9(10H)-蒽酮的体外DNA相互作用摘要:异亚芳基-9(10 H)-蒽酮在人细胞系中的合成,细胞毒性评估和体外DNA相互作用DOI:10.1002/ejoc.201701500
-
作为产物:描述:10-氯蒽-9-甲醇 在 2-碘酰基苯甲酸 作用下, 以 neat (no solvent) 为溶剂, 反应 3.0h, 以91%的产率得到10-氯-9-蒽甲醛参考文献:名称:IBX在球磨过程中在无溶剂条件下有效工作†摘要:IBX(2-碘氧基苯甲酸),发现于1893年,是合成化学中的一种氧化剂,由于其在高温下的爆炸性以及在除DMSO之外的普通有机溶剂中的溶解性差而被广泛使用而受到阻碍。自1983年发现Dess-Martin Periodinane以来,已经报道了几种改进的IBX系统。但是,在球磨条件下,IBX可以在环境温度和无溶剂条件下有效地利用各种有机功能。另外,反应产生的废IBA(2-碘代苯甲酸)也就地在接下来的步骤中,使用过硫酸氢钾将其氧化成IBX,因此通过保持其效率(在15个循环后仅损失约6%)而重复用于多个循环。这项工作概述了一种非常经济的合成方法,该方法克服了使用IBX的问题,可以有效地以克为单位且以非爆炸方式进行。DOI:10.1039/c4ra00415a
文献信息
-
Highly efficient carbazolyl-derived phosphine ligands: application to sterically hindered biaryl couplings作者:Sheung Chun To、Fuk Yee KwongDOI:10.1039/c1cc10708a日期:——A new family of phosphine ligands bearing a bulky carbazolyl scaffold is described. With the combination of ligand 2a and Pd(OAc)2, difficult tri-ortho-substituted biaryl couplings are accomplished smoothly. In particular, the catalyst loading as low as 0.02 mol% of Pd for non-activated 2,6-disubstituted aryl chloride coupling can be achieved.
-
In Silico Driven Design and Synthesis of Rhodanine Derivatives as Novel Antibacterials Targeting the Enoyl Reductase InhA作者:Liudas Slepikas、Gianpaolo Chiriano、Remo Perozzo、Sébastien Tardy、Agata Kranjc、Ophélie Patthey-Vuadens、Hajer Ouertatani-Sakouhi、Sébastien Kicka、Christopher F. Harrison、Tiziana Scrignari、Karl Perron、Hubert Hilbi、Thierry Soldati、Pierre Cosson、Eduardas Tarasevicius、Leonardo ScapozzaDOI:10.1021/acs.jmedchem.5b01620日期:2016.12.22computational studies. Their antimicrobial activity was assessed against Mycobacterium marinum (Mm) (a model for Mtb), Pseudomonas aeruginosa (Pa), Legionella pneumophila (Lp), and Enterococcus faecalis (Ef) by using anti-infective, antivirulence, and antibiotic assays. Nineteen out of 34 compounds reduced Mm virulence at 10 μM. 33 exhibited promising antibiotic activity against Mm with a MIC of 0.21 μM and showed在这里,我们报告针对结核分枝杆菌结核(Mtb)反-2-烯酰基酰基载体蛋白还原酶(InhA)的4-噻唑烷酮(若丹宁)衍生物的设计,合成和生物学评估。在罗丹宁环第5位具有庞大芳族取代基且在N -3位具有色氨酸残基的化合物对InhA的活性最高,IC 50值为2.7至30μM。实验数据显示与计算研究一致的相关性。他们的抗菌活性评估了对结核分枝杆菌(Mm)(铜绿假单胞菌(Mtb)的模型)。Pa),嗜肺军团菌(Lp)和粪肠球菌(Ef),方法是使用抗感染,抗毒力和抗生素检测方法。34种化合物中有19种降低了10μM的Mm毒力。33的MIC为0.21μM,对Mm表现出有希望的抗生素活性,在30μM的抗感染试验中,Lp的生长降低了89%。32显示对Ef的高抗生素活性,MIC为0.57μM。
-
Nucleophilic difluoromethylation and difluoromethylenation using bromodifluoromethyl phenyl sulfone作者:G.K. Surya Prakash、Ying Wang、Jinbo Hu、George A. OlahDOI:10.1016/j.jfluchem.2005.07.011日期:2005.10found to be an effective electron-transfer agent that promoted the reactions of bromodifluoromethyl phenyl sulfone with aldehydes to give structurally diverse (benzenesulfonyl)difluoromethylated alcohols in good yield, which can be further transformed into difluoromethyl alcohols and 1,1-difluoro-1-alkenes via reductive desulfonylation and Julia olefination, respectively.
-
Catalytic α-Selective Deuteration of Styrene Derivatives作者:Thomas R. Puleo、Alivia J. Strong、Jeffrey S. BandarDOI:10.1021/jacs.8b12874日期:2019.1.30We report an operationally simple protocol for the catalytic α-deuteration of styrenes. This process proceeds via the base-catalyzed reversible addition of methanol to styrenes in DMSO -d6 solvent. The concentration of methanol is shown to be critical for high yields and selectivities over multiple competing side reactions. The synthetic utility of α-deuterated styrenes for accessing deuterium-labeled
-
CuIBiOI is an efficient novel catalyst in Ullmann-type CN couplings with wide scope—A rare non-photocatalyic application作者:Gábor Varga、Marianna Kocsis、Ákos Kukovecz、Zoltán Kónya、Igor Djerdj、Pál Sipos、István PálinkóDOI:10.1016/j.mcat.2020.111072日期:2020.9Ullmann-type CN coupling reactions between aryl halides and aqueous ammonia, its catalytic capabilities were tested. The effects of solvents, added base and catalyst loading as well as reaction time and reaction temperature were scrutinized, and a green way for the reaction was identified. The recyclability of the catalyst without the loss of activity and its general applicability for a wide range of aryl halides
表征谱图
-
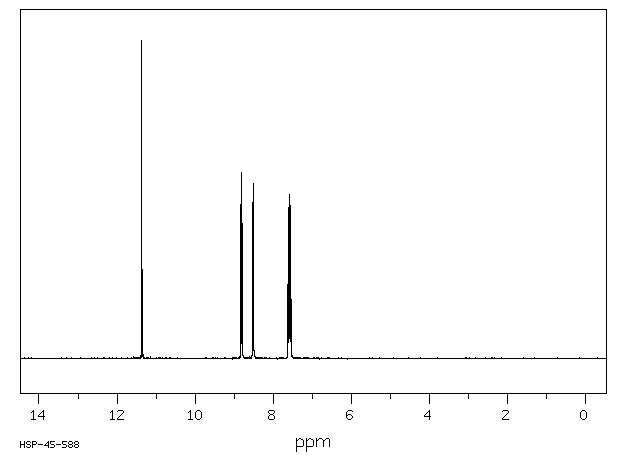
氢谱1HNMR
-
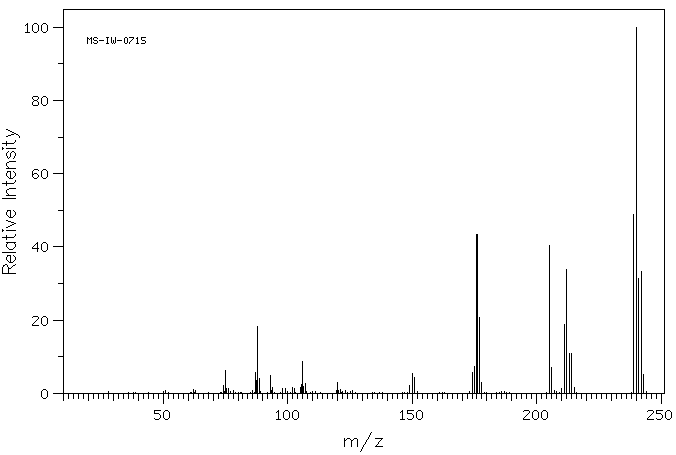
质谱MS
-
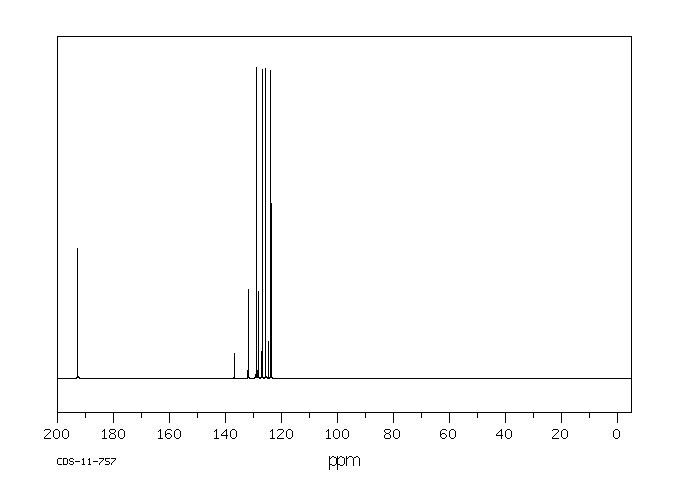
碳谱13CNMR
-
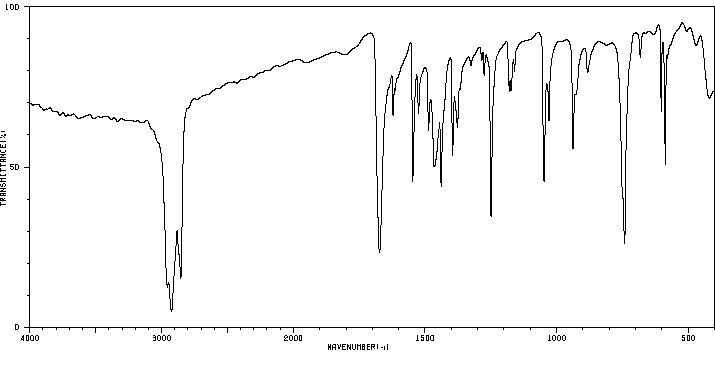
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62