15-甲基十六烷酸甲酯 | 6929-04-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:326.5±10.0 °C(Predicted)
-
密度:0.863±0.06 g/cm3(Predicted)
-
溶解度:氯仿:可溶;乙醇:可溶
-
保留指数:1974;1970;1974;1975.6
-
稳定性/保质期:
存在于烤烟烟叶和烟气中。
计算性质
-
辛醇/水分配系数(LogP):8.2
-
重原子数:20
-
可旋转键数:15
-
环数:0.0
-
sp3杂化的碳原子比例:0.944
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
WGK Germany:3
-
海关编码:2915900090
SDS
制备方法与用途
- 烟草: FC,9。
- 烟草:FC,9。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 15-甲基十六烷酸 isomargaric acid 1603-03-8 C17H34O2 270.456
反应信息
-
作为反应物:描述:参考文献:名称:Arosenius et al., Arkiv foer Kemi, 1949, vol. 26 A, # 19, p. 10摘要:DOI:
-
作为产物:描述:参考文献:名称:DOUMENO, P.;GUILIANO, M.;BERTRAND, J. C.;MILLE, G., APPL. SPECTROSC., 44,(1990) N, C. 1355-1359摘要:DOI:
文献信息
-
Insecticidal Activity of Extracts, Fractions, and Pure Molecules of Cissampelos pareira Linnaeus against Aphid, Aphis craccivora Koch作者:Surekha Kumari、Shudh Kirti Dolma、Anmol、Upendra Sharma、S. G. Eswara ReddyDOI:10.3390/molecules27030633日期:——
Aphis craccivora Koch is a polyphagous and major pest of leguminous crops causing significant damage by reducing the yield. Repeated application of synthetic insecticides for the control of aphids has led to development of resistance. Therefore, the present study aimed to screen the insecticidal activity of root/stem extracts/fractions, and pure molecules from Cissampelos pareira Linnaeus against A. craccivora for identification of lead(s). Among root extract/fractions, the n-hexane fraction was found most effective (LC50 = 1828.19 mg/L) against A. craccivora, followed by parent extract (LC50 = 2211.54 mg/L). Among stem extract/fractions, the n-hexane fraction (LC50 = 1246.92 mg/L) was more effective than the water and n-butanol fractions. Based on GC and GC-MS analysis, among different compounds identified in the n-hexane fraction of root and stem, ethyl palmitate (known to possess insecticidal activity) was present in the highest concentration (24.94 to 52.95%) in both the fractions. Among pure molecules, pareirarineformate was found most effective (LC50 = 1491.93 mg/L) against A. craccivora, followed by cissamine (LC50 = 1556.31 mg/L). Parent extract and fractions of C. pareira possess promising activity against aphid. Further, field bio-efficacy studies are necessary to validate the current findings for the development of botanical formulation.
Aphis craccivora Koch是一种多食性和主要的豆科作物害虫,通过减少产量造成显著损害。反复使用合成杀虫剂来控制蚜虫已经导致了抗药性的产生。因此,本研究旨在筛选来自Cissampelos pareira Linnaeus的根/茎提取物/分馏物和纯分子的杀虫活性,以识别潜在的领先物质。在根提取物/分馏物中,正己烷分馏物对A. craccivora的效果最好(LC50 = 1828.19 mg/L),其次是母体提取物(LC50 = 2211.54 mg/L)。在茎提取物/分馏物中,正己烷分馏物(LC50 = 1246.92 mg/L)比水和正丁醇分馏物更有效。基于GC和GC-MS分析,在根和茎的正己烷分馏物中识别出的不同化合物中,乙酸乙酯(已知具有杀虫活性)在两个分馏物中的浓度最高(24.94至52.95%)。在纯分子中,pareirarineformate对A. craccivora的效果最好(LC50 = 1491.93 mg/L),其次是cissamine(LC50 = 1556.31 mg/L)。C. pareira的母体提取物和分馏物具有很好的杀虫活性。进一步的田间生物效力研究有必要验证当前的发现以开发植物制剂。 -
2' ,5' -Oligoadenylate analogs申请人:Koizumi Makoto公开号:US20050261235A1公开(公告)日:2005-11-24A 2-5A analog represented by the formula (1): wherein m is 0 or 1; n is 0 to 2; R 1 represents an alkoxy group having from 1 to 6 carbon atoms which may be substituted, an unprotected mercapto group, a mercapto group protected by a nucleic acid synthesis protecting group, or an alkylthio group having from 1 to 4 carbon atoms which may be substituted; R 2 , R 3 , R 4 , R 5 and R 6 represent an unprotected hydroxyl group, a hydroxyl group protected by a nucleic acid synthesis protecting group, an alkoxy group having from 1 to 6 carbon atoms which may be substituted, an unprotected mercapto group, a mercapto group protected by a nucleic acid synthesis protecting group, or an alkylthio group having from 1 to 4 carbon atoms which may be substituted; R 7 represents an oxygen atom, or a —O(CH 2 CH 2 O)q-group, wherein q is 2 to 6; R 8 represents a hydrogen atom, an alkyl group having from 1 to 6 carbon atoms which may be substituted, or a 5′-phosphorylated oligonucleotide analog which has one hydroxyl group removed from the 5′-phosphoric acid group; E 1 , E 2 , E 3 and E 4 represent a naturally occurring or modified nucleic acid unit, or a pharmacologically acceptable salt thereof.一种由公式(1)表示的2-5A模拟物,其中m为0或1;n为0至2;R1表示具有1至6个碳原子的烷氧基,可以被取代,未保护的巯基,通过核酸合成保护基保护的巯基,或者具有1至4个碳原子的烷硫基,可以被取代;R2、R3、R4、R5和R6表示未保护的羟基,通过核酸合成保护基保护的羟基,具有1至6个碳原子的烷氧基,可以被取代,未保护的巯基,通过核酸合成保护基保护的巯基,或者具有1至4个碳原子的烷硫基,可以被取代;R7表示氧原子,或者-O(CH2CH2O)q-基团,其中q为2至6;R8表示氢原子,具有1至6个碳原子的烷基,可以被取代,或者从5'-磷酸基团中去除一个羟基的5'-磷酸寡核苷酸类似物;E1、E2、E3和E4表示天然或修饰的核酸单元,或其药理学上可接受的盐。
-
Method of treating a tumor or a viral disease by administering a 2' , 5' -oligoadenylate analog申请人:Koizumi Makoto公开号:US20100035976A1公开(公告)日:2010-02-11A method of treating a tumor or a viral disease by administering to a human the following 2′,5′-oligoadenylate analog: Wherein m is 0; n is 0 or 1; R 1 is alkoxy substituted by hydroxyl, mercapto, alkylthio substituted by hydroxyl or X 1 —X 2 —X 3 —S—; R 2 , R 3 , R 4 , R 5 and R 6 are hydroxyl, mercapto, alkylthio substituted by hydroxyl or X 1 —X 2 —X 3 —S—; R 7 is oxygen, sulfur, —NH—, or —O(CH 2 CH 2 O)q-, wherein q is 2 to 6, or oxyalkyleneoxy; R 8 is hydrogen or a 5′-phosphorylated oligonucleotide which has one hydroxyl removed from the 5′-phosphoric acid; E 1 is K 2 ; E 2 is K 1 ; E 3 is K 2 or K 3 and E 4 is K 1 , K 2 or K 3 ; K 1 is K 2 is K 3 is B is adeninyl; A is alkylene; D is alkyl or alkenyl; X 1 is alkyl or phenyl; X 2 is —C(═O)O—, —OC(═O)— or —C(═O)S—; and X 3 is alkylene.一种通过向人体内注射以下2′,5′-寡腺苷酸类似物来治疗肿瘤或病毒性疾病的方法:其中m为0;n为0或1;R1为烷氧基,被羟基,巯基,被羟基的烷基硫代基或X1—X2—X3—S—取代的烷氧基;R2,R3,R4,R5和R6为羟基,巯基,被羟基的烷基硫代基或X1—X2—X3—S—;R7为氧,硫,—NH—,或—O(CH2CH2O)q-,其中q为2至6,或氧烷氧基;R8为氢或一个5′-磷酸寡核苷酸,其5′-磷酸上有一个羟基被去除;E1为K2;E2为K1;E3为K2或K3,E4为K1,K2或K3;K1,K2和K3为B为腺苷基;A为烷基;D为烷基或烯基;X1为烷基或苯基;X2为—C(═O)O—,—OC(═O)—或—C(═O)S—;X3为烷基。
-
NOVEL 2' ,5' -OLIGOADENYLIC ACID ANALOGUES申请人:Sankyo Company, Limited公开号:EP1568704A1公开(公告)日:2005-08-31A 2-5A analog represented by the formula (1): [wherein m is 0 or 1; n is 0 to 2; R1 represents an alkoxy group having from 1 to 6 carbon atoms which may have a substituent, a mercapto group, a mercapto group protected by a nucleic acid synthesis protecting group, or an alkylthio group having from 1 to 4 carbon atoms which may have a substituent; R2, R3, R4, R5 and R6 represent a hydroxyl group, a hydroxyl group protected by a nucleic acid synthesis protecting group, an alkoxy group having from 1 to 6 carbon atoms which may have a substituent, a mercapto group, a mercapto group protected by a nucleic acid synthesis protecting group, or an alkylthio group having from 1 to 4 carbon atoms which may have a substituent; R7 represents an oxygen atom, or a -O(CH2CH2O)q- group (q is 2 to 6); R8 represents a hydrogen atom, an alkyl group having from 1 to 6 carbon atoms which may have a substituent, or 5'-phosphorylated oligonucleotide analogs which do not have one hydroxyl group on a 5'-phosphoric acid group; E1, E2, E3 and E4 represent a naturally occurring or modified nucleic acid unit], and a pharmacologically acceptable salt thereof.由式(1)表示的 2-5A 类似物: [其中 m 为 0 或 1;n 为 0 至 2;R1 代表具有 1 至 6 个碳原子且可能具有取代基的烷氧基、巯基、受核酸合成保护基保护的巯基或具有 1 至 4 个碳原子且可能具有取代基的烷硫基;R2、R3、R4、R5 和 R6 代表羟基、受核酸合成保护基团保护的羟基、具有 1 至 6 个碳原子且可能具有取代基的烷氧基、巯基、受核酸合成保护基团保护的巯基或具有 1 至 4 个碳原子且可能具有取代基的烷硫基;R7代表氧原子,或-O(CH2CH2O)q-基团(q为2至6);R8代表氢原子、具有1至6个碳原子且可具有取代基的烷基,或在5'-磷酸基上不具有一个羟基的5'-磷酸化寡核苷酸类似物;E1、E2、E3和E4代表天然存在的或修饰的核酸单元],及其药理学上可接受的盐。
-
Arosenius et al., Arkiv foer Kemi, 1949, vol. 26 A, # 19, p. 3,5, 17作者:Arosenius et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
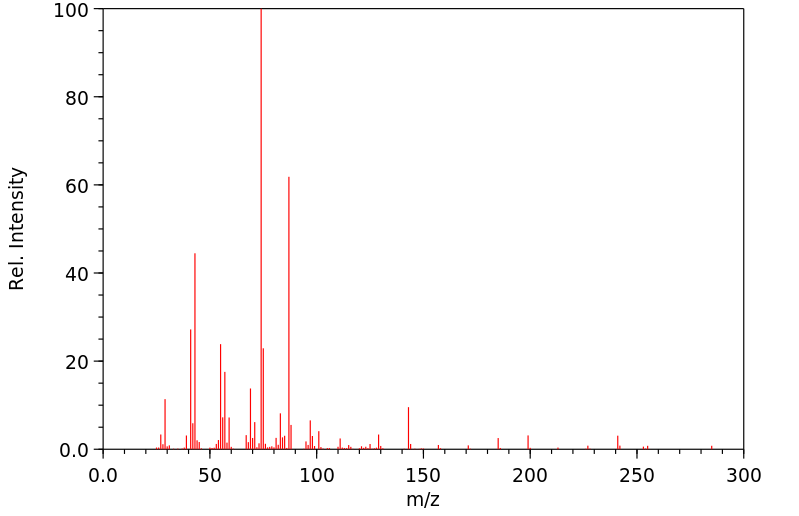
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息