2,5-二氰基吡啶 | 20730-07-8
中文名称
2,5-二氰基吡啶
中文别名
——
英文名称
Pyridine-2,5-dicarbonitrile
英文别名
2,5-Dicyanopyridine;2,5-DCP;Pyridin-2,5-dicarbonitril;5-cyanopyridine-2-carbonitrile
CAS
20730-07-8
化学式
C7H3N3
mdl
——
分子量
129.121
InChiKey
YXHPFSCDWSLLRT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:111-112 °C
-
沸点:110 °C(Press: 3 Torr)
-
密度:1.25±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:60.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2933399090
-
危险性防范说明:P280,P305+P351+P338,P310
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:存放于室温、干燥且密封的环境中。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-炔基烟腈 6-ethynylnicotinonitrile 1211588-35-0 C8H4N2 128.133 5-乙基吡啶-2-甲腈 5-ethyl-pyridine-2-carbonitrile 14178-13-3 C8H8N2 132.165 2,5-二甲基吡啶 2,5-dimethylpyridine 589-93-5 C7H9N 107.155 —— 2-((2-trimethylsilyl)acetylenyl)-5-cyanopyridine 1214278-89-3 C11H12N2Si 200.315 5-乙基-2-甲基-吡啶 5-ethyl-2-methyl-pyridine 104-90-5 C8H11N 121.182 2-甲基-5-乙烯基吡啶 2-methyl-5-vinylpyridine 140-76-1 C8H9N 119.166 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氰基吡啶 2-Cyanopyridine 100-70-9 C6H4N2 104.111
反应信息
-
作为反应物:参考文献:名称:Discovery and characterization of AZD9272 and AZD6538—Two novel mGluR5 negative allosteric modulators selected for clinical development摘要:AZD9272 and AZD6538 are two novel mGluR5 negative allosteric modulators selected for further clinical development. An initial high-throughput screening revealed leads with promising profiles, which were further optimized by minor, yet indispensable, structural modifications to bring forth these drug candidates. Advantageously, both compounds may be synthesized in as little as one step. Both are highly potent and selective for the human as well as the rat mGluR5 where they interact at the same binding site than MPEP. They are orally available, allow for long interval administration due to a high metabolic stability and long half-lives in rats and permeate the blood brain barrier to a high extent. AZD9272 has progressed into phase I clinical studies. (C) 2012 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2012.08.100
-
作为产物:描述:参考文献:名称:电子不足的杂芳烃盐:氧化中过氧化氢活化的有机催化工具摘要:制备了一系列的单取代的嘧啶鎓和吡嗪鎓三氟甲磺酸酯和3,5-二取代的吡啶鎓三氟甲磺酸酯,并使用硫氧化作为模型反应,对过氧化氢的简单氧化催化剂进行了测试。它们的催化效率在很大程度上取决于取代基的类型,并且对于具有吸电子基团的衍生物而言是显着的,其反应性与黄酮盐相当,后者是氧合作用的主要有机催化剂。由于它们的高稳定性和良好的可及性,4-(三氟甲基)嘧啶鎓和3,5-二硝基吡啶鎓三氟甲磺酸盐是选择的催化剂,并显示出催化脂肪族和芳香族硫化物氧化为亚砜的作用,定量转化率高,制备产率高,并且具有优异的性能。化学选择性。K R +值(p K R + <5)和较小的负还原电位(E red > -0.5 V)。在催化氧化过程中原位形成的过氧化氢加合物充当底物氧化剂。通过在B3LYP / 6-311 ++ g(d,p)水平上的计算获得的从这些杂环氢过氧化物到硫代苯甲醚的转移的吉布斯自由能表明,它们是比烷基氢过氧化DOI:10.1021/jo502865f
文献信息
-
[EN] IRIDIUM COMPLEXES FOR CELLULAR IMAGING<br/>[FR] COMPLEXES D'IRIDIUM POUR IMAGERIE CELLULAIRE申请人:UNIV SOUTH AUSTRALIA公开号:WO2018014078A1公开(公告)日:2018-01-25The present disclosure relates to methods and products for imaging or labelling cells. Certain embodiments of the present disclosure provide a method of intracellular imaging of a cell. The method comprises exposing the cell to a complex comprising a phenylpyridine iridium (III) (and/or a functional derivative thereof) and a tetrazolato compound and imaging the complex in the cell.
-
Cleavage of the Carbon-Carbon Triple Bonds of Arylacetylenes for the Synthesis of Arylnitriles without a Metal Catalyst作者:Yuanguang Lin、Qiuling SongDOI:10.1002/ejoc.201600438日期:2016.6Cleavage of the carbon–carbon triple bonds of alkynes was achieved, which led to the synthesis of arylnitriles under transition-metal-free conditions. A vast range of terminal alkyne substrates underwent this reaction to provide the corresponding nitriles in moderate to good yields with good functional group tolerance.
-
Heteropolycyclic compounds and their use as metabotropic glutamate receptor antagonists申请人:——公开号:US20030055085A1公开(公告)日:2003-03-20The present invention provides compounds and pharmaceutical compositions that act as antagonists at metabotropic glutamate receptors, and that are useful for treating neurological diseases and disorders. Methods of preparing the compounds also are disclosed.本发明提供了作为代谢型谷氨酸受体拮抗剂的化合物和药物组合物,用于治疗神经系统疾病和障碍。还公开了制备这些化合物的方法。
-
A Homogeneous Method for the Conveniently Scalable Palladium- and Nickel-Catalyzed Cyanation of Aryl Halides作者:Finn Burg、Julian Egger、Johannes Deutsch、Nicolas GuimondDOI:10.1021/acs.oprd.6b00229日期:2016.8.19Homogeneous conditions for the palladium-catalyzed cyanation of aryl halides were developed. This new system features a broad scope of aryl chlorides and bromides, uses 2-propanol or 1-butanol as solvent, and is readily scalable. The same conditions can also provide simple benzonitriles using the recently developed (TMEDA)NiCl(o-tolyl) precatalyst in conjunction with 1,1′-bis(diphenylphosphino)ferrocene
-
Bis(4-hydroxythiazoles): Novel Functional and Switchable Fluorophores作者:Dieter Weiß、Rainer Beckert、Eric Täuscher、Lorena Calderón-Ortiz、Helmar GörlsDOI:10.1055/s-0030-1260670日期:2011.7A series of 2,2′- and 5,5′-bis(4-hydroxythiazoles) was synthesized according to Hantzsch synthesis. Both phenols and their corresponding anions display a strong fluorescence in the visible spectrum, whereas the emission is shifted bathochromically upon deprotonation. This easy switch makes them suitable for widespread applications, mainly in analysis and supramolecular chemistry. Due to their 1,4-diazadiene substructure, the 2,2′-bithiazoles possess prerequisites for the complexation of metals, however, instead of a copper complex, a dimer which constitutes the 5,5′-C-C coupled product was isolated. In addition, tris(thiazoles) could be constructed.
表征谱图
-
氢谱1HNMR
-
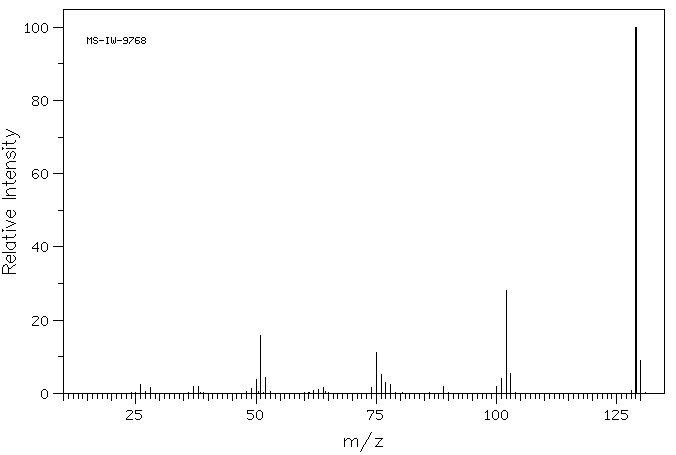
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-